Organotitanium compound
Encyclopedia

Organometallic chemistry
Organometallic chemistry is the study of chemical compounds containing bonds between carbon and a metal. Since many compounds without such bonds are chemically similar, an alternative may be compounds containing metal-element bonds of a largely covalent character...
contain carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
to titanium
Titanium
Titanium is a chemical element with the symbol Ti and atomic number 22. It has a low density and is a strong, lustrous, corrosion-resistant transition metal with a silver color....
chemical bond
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
s. Organotitanium chemistry is the science of organotitanium compounds describing their physical properties, synthesis and reactions. They are reagents in organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
and are involved in major industrial processes.
Brief history
Although the first attempt at an organotitanium compound dates back to 1861, it took until 1953 for the first such compound to arrive. In that year titanium phenyl-tri(isopropoxide) was prepared by Herman and Nelson from titanium isopropoxide, phenyllithiumPhenyllithium
Phenyllithium is an organometallic agent with the empirical formula C6H5Li. It is most commonly used as a metalating agent in organic syntheses and a substitute for Grignard reagents for introducing phenyl groups in organic syntheses...
and titanium(IV) chloride. Titanocene dichloride
Titanocene dichloride
Titanocene dichloride is the organotitanium compound with the formula 2TiCl2, commonly abbreviated as Cp2TiCl2. This metallocene is a common reagent in organometallic and organic synthesis. It exists as a bright red solid that slowly hydrolyzes in air...
was invented in 1954, and the first methyltitanium compounds were introduced in 1959. Ziegler-Natta catalyst
Ziegler-Natta catalyst
A Ziegler–Natta catalyst is a catalyst used in the synthesis of polymers of 1-alkenes . Three types of Ziegler–Natta catalysts are currently employed:* Solid and supported catalysts based on titanium compounds...
s utilizing titanium-based catalysts soon followed as a major commercial application for which the Nobel Prize in Chemistry
Nobel Prize in Chemistry
The Nobel Prize in Chemistry is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outstanding contributions in chemistry, physics, literature,...
of 1963 was awarded.
Properties
The titanium electron configurationElectron configuration
In atomic physics and quantum chemistry, electron configuration is the arrangement of electrons of an atom, a molecule, or other physical structure...
([Ar]3d24s2) resembles that of carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
and like carbon the +4 oxidation state
Oxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
dominates and like carbon compounds, those of titanium have a tetrahedral molecular geometry
Tetrahedral molecular geometry
In a tetrahedral molecular geometry a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are cos−1 ≈ 109.5° when all four substituents are the same, as in CH4. This molecular geometry is common throughout the first...
. Thus, the boiling point
Boiling point
The boiling point of an element or a substance is the temperature at which the vapor pressure of the liquid equals the environmental pressure surrounding the liquid....
s of TiCl4 and CCl4 are very similar. Titanium is however a much larger element than carbon, reflected by the Ti-C bond length
Bond length
- Explanation :Bond length is related to bond order, when more electrons participate in bond formation the bond will get shorter. Bond length is also inversely related to bond strength and the bond dissociation energy, as a stronger bond will be shorter...
es being about 30% longer, e.g. 210 pm in tetrabenzyltitanium vs a typical C-C bond of 155 pm. Simple tetraalkyltitanium compounds however are not typically stable, owing to the large size of titanium and the electron-deficient nature of its tetrahdral complexes. More abundant and more useful than the simple tetraalkyl compounds are organic derivatives with alkoxide and cyclopentadienyl coligands. Titanium is capable of forming complexes with high coordination number
Coordination number
In chemistry and crystallography, the coordination number of a central atom in a molecule or crystal is the number of its nearest neighbours. This number is determined somewhat differently for molecules and for crystals....
s.
In terms of oxidation states, most organotitanium chemistry, in solution at least, focuses on derivatives of Ti(IV). Ti(II) compounds are rarer, examples being titanocene dicarbonyl
Titanocene dicarbonyl
Dicarbonylbistitanium is the chemical compound with the formula 2Ti2, abbreviated Cp2Ti2. This maroon-coloured, air-sensitive species is soluble in aliphatic and aromatic solvents...
and Ti(CH3)2(dmpe
1,2-Bis(dimethylphosphino)ethane
1,2-Bisethane is a diphosphine ligand in coordination chemistry. It can be synthesised by the reaction of methylmagnesium iodide with 1,2-bisethane:...
)2. [Ti(CO)6]2- is formally a complex of Ti(-II). Although Ti(III) is involved in Ziegler-Natta catalysis, the organic derivatives of Ti(III) are not common.
Due to the low electronegativity
Electronegativity
Electronegativity, symbol χ , is a chemical property that describes the tendency of an atom or a functional group to attract electrons towards itself. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus...
of titanium, Ti-C bonds are polarized toward carbon. Consequently, alkyl ligands in many titanium compounds are nucleophilic. Titanium is characteristically oxophilic, which presents challenges to handling these compounds, which require air-free technique
Air-free technique
Air-free techniques refer to a range of manipulations in the chemistry laboratory for the handling of compounds that are air-sensitive. These techniques prevent the compounds from reacting with components of air, usually water and oxygen; less commonly carbon dioxide and nitrogen...
s. On the other hand, high oxophilicity means that titanium alklyls are effective for abstracting or exchanging organyl ligands for oxo groups, as discussed below.
Chemistry
Organotitanium compounds are important reagents in organic chemistryOrganic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
. Some reagents include the following.
- The Ziegler-Natta catalystZiegler-Natta catalystA Ziegler–Natta catalyst is a catalyst used in the synthesis of polymers of 1-alkenes . Three types of Ziegler–Natta catalysts are currently employed:* Solid and supported catalysts based on titanium compounds...
(1954) is obtained from titanium(III) chlorideTitanium(III) chlorideTitanium chloride is the inorganic compound with the formula TiCl3. At least four distinct species have this formula; additionally hydrated derivatives are known...
and diethylaluminium chloride and important in ethyleneEthyleneEthylene is a gaseous organic compound with the formula . It is the simplest alkene . Because it contains a carbon-carbon double bond, ethylene is classified as an unsaturated hydrocarbon. Ethylene is widely used in industry and is also a plant hormone...
polymerizationPolymerizationIn polymer chemistry, polymerization is a process of reacting monomer molecules together in a chemical reaction to form three-dimensional networks or polymer chains...
. - Methyl titanium trichloride CH3TiCl3 (1959) is a nonbasic nucleophilic reagent. It can be prepared by reacting titanium(IV) chloride with dimethylzinc in dichloromethaneDichloromethaneDichloromethane is an organic compound with the formula CH2Cl2. This colorless, volatile liquid with a moderately sweet aroma is widely used as a solvent. Although it is not miscible with water, it is miscible with many organic solvents...
at -78 °C. It is used in nucleophilic additionNucleophilic additionIn organic chemistry, a nucleophilic addition reaction is an addition reaction where in a chemical compound a π bond is removed by the creation of two new covalent bonds by the addition of a nucleophile....
of methyl groups to carbonyl compounds and in SN1SN1 reactionThe SN1 reaction is a substitution reaction in organic chemistry. "SN" stands for nucleophilic substitution and the "1" represents the fact that the rate-determining step is unimolecular...
methylation of alkyl halides. Methyltriisopropoxytitanium is a related reagent prepared in situ from titanium isopropoxide, titanium(IV) chloride and methyllithium - The Kulinkovich reactionKulinkovich reactionThe Kulinkovich reaction describes the organic synthesis of cyclopropanols via reaction of esters with dialkyldialkoxytitanium reagents, generated in situ from Grignard reagents bearing hydrogen in beta-position and titanium alkoxides such as titanium isopropoxide. This reaction was discovered by...
is a cyclopropanation method starting from a Grignard reagent and an ester. The first step is transmetallation forming a dialkyltitanium intermediate. - Lombardo's reagent is a carbenoidCarbenoidIn chemistry a carbenoid is a reactive intermediate that shares reaction characteristics with a carbene. In the Simmons-Smith reaction the carbenoid intermediate is a zinc / iodine complex that takes the form of...
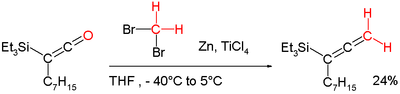
methylenation reagent (see Tebbe reagent below)., which is an aged version of the Dibromomethane-Zinc-Titanium(IV) Chloride reagent can for example be applied in a conversion of a keteneKeteneA ketene is an organic compound of the form R'RC=C=O. The term is also used specifically to mean ethenone, the simplest ketene, where R' and R are hydrogen atoms.Ketenes were first studied as a class by Hermann Staudinger.-Formation:...
into an alleneAlleneAn allene is a compound in which one carbon atom has double bonds with each of its two adjacent carbon centres. Allenes are classified as polyenes with cumulated dienes. The parent compound of allene is propadiene. Compounds with an allene-type structure but with more than three carbon atoms are...
:
Titanocene derivatives
A particularly rich area of organotitanium chemistry are derivatives of titanocene dichloride. Early work on "titanocene" itself eventually revealed that this species was a fulvaleneFulvalene
A fulvalene is a hydrocarbon obtained by formally cross-conjugating two rings through a common exocyclic double bond. The name is derived from the similarly structured fulvenes which lack one ring...
complex, and this discovery led to many innovations on cyclopentadienyl complexes of titanium.
Tebbe's reagent
Tebbe's reagent
The Tebbe reagent is the organometallic compound with the formula 2TiCH2ClAl2. It used in the methylenation of carbonyl compounds, that is it converts organic compounds containing the R2C=O group into the related R2C=CH2 derivative...
(1978) is prepared from titanocene dichloride
Titanocene dichloride
Titanocene dichloride is the organotitanium compound with the formula 2TiCl2, commonly abbreviated as Cp2TiCl2. This metallocene is a common reagent in organometallic and organic synthesis. It exists as a bright red solid that slowly hydrolyzes in air...
and trimethylaluminium
Trimethylaluminium
Trimethylaluminium is the chemical compound with the formula Al26, abbreviated as Al2Me6, 2 or the abbreviation TMA. This pyrophoric, colorless liquid is an industrially important organoaluminium compound...
. It is used as a methylenation
Methylene
Methylene is a chemical species in which a carbon atom is bonded to two hydrogen atoms. Three different possibilities present themselves:* the -CH2- substituent group: e.g., dichloromethane ....
agent for carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
compounds (converstion of R2C=O to R2C=CH2). It is an alternative for Wittig reagents when the carbonyl group is sterically challenged or when it easily forms the enol
Enol
Enols are alkenes with a hydroxyl group affixed to one of the carbon atoms composing the double bond. Alkenes with a hydroxyl group on both sides of the double bond are called enediols. Deprotonated anions of enols are called enolates...
. Tebbe's reagent adds simple alkenes to give titanocyclobutanes, which can be regarded as stable olefin metathesis
Olefin metathesis
Olefin metathesis or transalkylidenation is an organic reaction that entails redistribution of alkylene fragments by the scission of carbon - carbon double bonds in olefins . Its advantages include the creation of fewer sideproducts and hazardous wastes. Yves Chauvin, Robert H. Grubbs, and Richard R...
intermediates. These compounds are reagents in itself such as 1,1-bis(cyclopentadienyl)-3,3-dimethyltitanocyclobutane, the adduct of Tebbe's reagent with isobutene catalysed with 4-dimethylaminopyridine.
The Petasis reagent
Petasis reagent
The Petasis reagent is dimethyl titanocene, Cp2TiMe2, readily prepared by the reaction of methylmagnesium chloride or methyllithium with titanocene dichloride:...
or dimethyl titanocene (1990) is prepared from titanocene dichloride
Titanocene dichloride
Titanocene dichloride is the organotitanium compound with the formula 2TiCl2, commonly abbreviated as Cp2TiCl2. This metallocene is a common reagent in organometallic and organic synthesis. It exists as a bright red solid that slowly hydrolyzes in air...
and methyllithium in diethyl ether
Diethyl ether
Diethyl ether, also known as ethyl ether, simply ether, or ethoxyethane, is an organic compound in the ether class with the formula . It is a colorless, highly volatile flammable liquid with a characteristic odor...
. Compared to Tebbe's reagent it is easier to prepare and easier to handle. It is also a methylenation reagent.