Physical organic chemistry
Encyclopedia
Physical organic chemistry is the study of the interrelationships between structure
and reactivity in organic molecules. It can be seen as the study of organic chemistry
using tools of physical chemistry
such as chemical equilibrium
, chemical kinetics
, thermochemistry
, and quantum chemistry
. The term "physical organic chemistry" is commonly attributed to Louis Hammett, who used it as a title for a book in 1940.
The two main themes in physical organic chemistry are structure and reactivity. The study of structure starts from chemical bonding, with special emphasis on the stability of organic molecules due to factors such as steric strain and aromaticity
. Other topics in structure include stereochemistry
and conformational analysis. Supramolecular structure is also considered in terms of intermolecular forces including hydrogen bonding. Finally, the acid-base chemistry of the molecules is studied in terms of structure, based on resonance
and inductive
effects and through the use of linear free-energy relations
.
 The study of reactivity focuses on the mechanisms
The study of reactivity focuses on the mechanisms
of organic reactions. It uses chemical kinetics, spectroscopy
, isotope effect
s, and quantum chemistry to determine the sequence of elementary steps involved in a reaction. These elementary steps can be classified in a few major classes: addition
, elimination
, substitution
, and pericyclic reaction
s. The mechanisms are commonly expressed in terms of "electron pushing" and potential energy surface
s. Other major topics are photochemistry
, the effect of light on the reactivity of organic molecules, and solvent effects
on organic reactions.
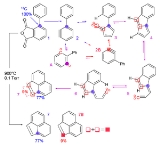
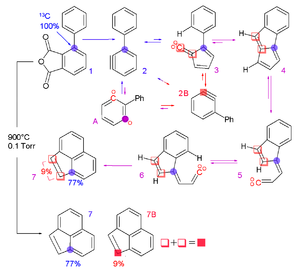
Structure and reactivity are both involved in the study of reaction intermediate
s—the transient species involved in reaction mechanisms. The main types of intermediates of interest are carbocation
s, carbanion
s, free radicals, and carbene
s. Usually, these intermediates are not isolated, but their presence is inferred from stereochemical evidence, spectroscopy, or through the use of chemical trap
s. In some cases, however, it is possible to isolate these types of molecules at very low temperatures (cryochemistry
) or via matrix isolation
. It is also possible to create specific derivatives
that are stabilized through chemical means such as resonance, as in the case of the triphenylmethyl radical
.
Chemical structure
A chemical structure includes molecular geometry, electronic structure and crystal structure of molecules. Molecular geometry refers to the spatial arrangement of atoms in a molecule and the chemical bonds that hold the atoms together. Molecular geometry can range from the very simple, such as...
and reactivity in organic molecules. It can be seen as the study of organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
using tools of physical chemistry
Physical chemistry
Physical chemistry is the study of macroscopic, atomic, subatomic, and particulate phenomena in chemical systems in terms of physical laws and concepts...
such as chemical equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
, chemical kinetics
Chemical kinetics
Chemical kinetics, also known as reaction kinetics, is the study of rates of chemical processes. Chemical kinetics includes investigations of how different experimental conditions can influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition...
, thermochemistry
Thermochemistry
Thermochemistry is the study of the energy and heat associated with chemical reactions and/or physical transformations. A reaction may release or absorb energy, and a phase change may do the same, such as in melting and boiling. Thermochemistry focuses on these energy changes, particularly on the...
, and quantum chemistry
Quantum chemistry
Quantum chemistry is a branch of chemistry whose primary focus is the application of quantum mechanics in physical models and experiments of chemical systems...
. The term "physical organic chemistry" is commonly attributed to Louis Hammett, who used it as a title for a book in 1940.
The two main themes in physical organic chemistry are structure and reactivity. The study of structure starts from chemical bonding, with special emphasis on the stability of organic molecules due to factors such as steric strain and aromaticity
Aromaticity
In organic chemistry, Aromaticity is a chemical property in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. The earliest use of the term was in an article by August...
. Other topics in structure include stereochemistry
Stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules....
and conformational analysis. Supramolecular structure is also considered in terms of intermolecular forces including hydrogen bonding. Finally, the acid-base chemistry of the molecules is studied in terms of structure, based on resonance
Resonance (chemistry)
In chemistry, resonance or mesomerism is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by one single Lewis formula...
and inductive
Inductive effect
In chemistry and physics, the inductive effect is an experimentally observable effect of the transmission of charge through a chain of atoms in a molecule by electrostatic induction...
effects and through the use of linear free-energy relations
Free-energy relationship
In physical organic chemistry, a free-energy relationship or linear Gibbs energy relation relates the logarithm of a reaction rate constant or equilibrium constant for one series of reactions with the logarithm of the rate or equilibrium constant for a related series of reactions...
.

Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
of organic reactions. It uses chemical kinetics, spectroscopy
Spectroscopy
Spectroscopy is the study of the interaction between matter and radiated energy. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, e.g., by a prism. Later the concept was expanded greatly to comprise any interaction with radiative...
, isotope effect
Isotope effect
Isotope effect can refer to:* Kinetic isotope effect* Magnetic isotope effect* Superconductive transition temperature varying by isotope atomic weight; See BCS theory#Successes of the BCS theory...
s, and quantum chemistry to determine the sequence of elementary steps involved in a reaction. These elementary steps can be classified in a few major classes: addition
Addition reaction
An addition reaction, in organic chemistry, is in its simplest terms an organic reaction where two or more molecules combine to form a larger one....
, elimination
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
, substitution
Substitution reaction
In a substitution reaction, a functional group in a particular chemical compound is replaced by another group. In organic chemistry, the electrophilic and nucleophilic substitution reactions are of prime importance...
, and pericyclic reaction
Pericyclic reaction
In organic chemistry, a pericyclic reaction is a type of organic reaction wherein the transition state of the molecule has a cyclic geometry, and the reaction progresses in a concerted fashion. Pericyclic reactions are usually rearrangement reactions...
s. The mechanisms are commonly expressed in terms of "electron pushing" and potential energy surface
Potential energy surface
A potential energy surface is generally used within the adiabatic or Born–Oppenheimer approximation in quantum mechanics and statistical mechanics to model chemical reactions and interactions in simple chemical and physical systems...
s. Other major topics are photochemistry
Photochemistry
Photochemistry, a sub-discipline of chemistry, is the study of chemical reactions that proceed with the absorption of light by atoms or molecules.. Everyday examples include photosynthesis, the degradation of plastics and the formation of vitamin D with sunlight.-Principles:Light is a type of...
, the effect of light on the reactivity of organic molecules, and solvent effects
Solvent effects
In chemistry, Solvent effects is the group of effects that a solvent has on chemical reactivity. Solvents can have an effect on solubility, stability and reaction rates and choosing the appropriate solvent allows for thermodynamic and kinetic control over a chemical reaction.-Effects on...
on organic reactions.
Structure and reactivity are both involved in the study of reaction intermediate
Reaction intermediate
A reaction intermediate or an intermediate is a molecular entity that is formed from the reactants and reacts further to give the directly observed products of a chemical reaction. Most chemical reactions are stepwise, that is they take more than one elementary step to complete...
s—the transient species involved in reaction mechanisms. The main types of intermediates of interest are carbocation
Carbocation
A carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
s, carbanion
Carbanion
A carbanion is an anion in which carbon has an unshared pair of electrons and bears a negative charge usually with three substituents for a total of eight valence electrons. The carbanion exists in a trigonal pyramidal geometry. Formally a carbanion is the conjugate base of a carbon acid.where B...
s, free radicals, and carbene
Carbene
In chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is RR'C:, but the carbon can instead be double-bonded to one group. The term "carbene" may also merely refer to the compound H2C:, also called...
s. Usually, these intermediates are not isolated, but their presence is inferred from stereochemical evidence, spectroscopy, or through the use of chemical trap
Chemical trap
In chemistry, a chemical trap is a chemical compound that is used to detect a certain molecule when* The concentration of this molecule is very small and below detection limit...
s. In some cases, however, it is possible to isolate these types of molecules at very low temperatures (cryochemistry
Cryochemistry
Cryochemistry is the study of chemical interactions at extremely low temperatures. As such, it has overlaps with the field of condensed matter physics and some relevance within the field of astrochemistry....
) or via matrix isolation
Matrix Isolation
Matrix isolation is an experimental technique used in chemistry and physics which generally involves a material being trapped within an unreactive matrix. A host matrix is a continuous solid phase in which guest particles are embedded. The guest is said to be isolated within the host matrix...
. It is also possible to create specific derivatives
Derivative (chemistry)
In chemistry, a derivative is a compound that is derived from a similar compound by some chemical or physical process. In the past it was also used to mean a compound that can be imagined to arise from another compound, if one atom is replaced with another atom or group of atoms, but modern...
that are stabilized through chemical means such as resonance, as in the case of the triphenylmethyl radical
Triphenylmethyl radical
The triphenylmethyl radical is a persistent radical and the first-ever radical described in organic chemistry. It can be prepared by homolysis of triphenylmethyl chloride 1 by a metal like silver or zinc in benzene or diethyl ether. The radical 2 forms a chemical equilibrium with the quinoid type...
.

