
Transparency and translucency
Encyclopedia
Optics
Optics is the branch of physics which involves the behavior and properties of light, including its interactions with matter and the construction of instruments that use or detect it. Optics usually describes the behavior of visible, ultraviolet, and infrared light...
, transparency (also called pellucidity or diaphaneity) is the physical property
Physical property
A physical property is any property that is measurable whose value describes a physical system's state. The changes in the physical properties of a system can be used to describe its transformations ....
of allowing light to pass through a material; translucency (also called translucence or translucidity) only allows light to pass through diffusely. The opposite property is opacity. Transparent materials appear clear, with the overall appearance of one color, or any combination leading up to a brilliant spectrum of every color.
When light encounters a material, it can interact with it in several different ways. These interactions depend on the wavelength
Wavelength
In physics, the wavelength of a sinusoidal wave is the spatial period of the wave—the distance over which the wave's shape repeats.It is usually determined by considering the distance between consecutive corresponding points of the same phase, such as crests, troughs, or zero crossings, and is a...
of the light and the nature of the material. Light waves interact with an object by some combination of reflection
Reflection (physics)
Reflection is the change in direction of a wavefront at an interface between two differentmedia so that the wavefront returns into the medium from which it originated. Common examples include the reflection of light, sound and water waves...
, and transmittance
Transmittance
In optics and spectroscopy, transmittance is the fraction of incident light at a specified wavelength that passes through a sample. A related term is absorptance, or absorption factor, which is the fraction of radiation absorbed by a sample at a specified wavelength...
with refraction
Refraction
Refraction is the change in direction of a wave due to a change in its speed. It is essentially a surface phenomenon . The phenomenon is mainly in governance to the law of conservation of energy. The proper explanation would be that due to change of medium, the phase velocity of the wave is changed...
.
Some materials, such as plate glass and clean water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
, allow much of the light that falls on them to be transmitted, with little being reflected; such materials are called optically transparent. Many liquids and aqueous solutions are highly transparent. Absence of structural defects (voids, cracks, etc.) and molecular structure of most liquids are mostly responsible for excellent optical transmission.
Materials which do not allow the transmission of light are called opaque
Opacity (optics)
Opacity is the measure of impenetrability to electromagnetic or other kinds of radiation, especially visible light. In radiative transfer, it describes the absorption and scattering of radiation in a medium, such as a plasma, dielectric, shielding material, glass, etc...
. Many such substances have a chemical composition which includes what are referred to as absorption
Absorption (electromagnetic radiation)
In physics, absorption of electromagnetic radiation is the way by which the energy of a photon is taken up by matter, typically the electrons of an atom. Thus, the electromagnetic energy is transformed to other forms of energy for example, to heat. The absorption of light during wave propagation is...
centers. Many substances are selective in their absorption of white light
White Light
White Light may refer to:*Light with the color white*White Light , a 1980 novel by Rudy Rucke*White Light , 1971 album*White Light , 2010 album...
frequencies. They absorb certain portions of the visible spectrum
Visible spectrum
The visible spectrum is the portion of the electromagnetic spectrum that is visible to the human eye. Electromagnetic radiation in this range of wavelengths is called visible light or simply light. A typical human eye will respond to wavelengths from about 390 to 750 nm. In terms of...
, while reflecting others. The frequencies of the spectrum which are not absorbed are either reflected back or transmitted for our physical observation. This is what gives rise to color
Color
Color or colour is the visual perceptual property corresponding in humans to the categories called red, green, blue and others. Color derives from the spectrum of light interacting in the eye with the spectral sensitivities of the light receptors...
. The attenuation of light of all frequencies and wavelengths is due to the combined mechanisms of absorption and scattering.
Introduction
With regard to the absorption of light, primary material considerations include:- At the electronic level, absorption in the ultravioletUltravioletUltraviolet light is electromagnetic radiation with a wavelength shorter than that of visible light, but longer than X-rays, in the range 10 nm to 400 nm, and energies from 3 eV to 124 eV...
and visible (UV-Vis) portions of the spectrum depends on whether the electron orbitalsAtomic orbitalAn atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus...
are spaced (or "quantized") such that they can absorb a quantumQuantumIn physics, a quantum is the minimum amount of any physical entity involved in an interaction. Behind this, one finds the fundamental notion that a physical property may be "quantized," referred to as "the hypothesis of quantization". This means that the magnitude can take on only certain discrete...
of light (or photonPhotonIn physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force...
) of a specific frequencyFrequencyFrequency is the number of occurrences of a repeating event per unit time. It is also referred to as temporal frequency.The period is the duration of one cycle in a repeating event, so the period is the reciprocal of the frequency...
, and does not violate selection rules. For example, in most glasses, electrons have no available energy levels above them in range of that associated with visible light, or if they do, they violate selection rules, meaning there is no appreciable absorption in pure (undoped) glasses, making them ideal transparent materials for windows in buildings. - At the atomic or molecular level, physical absorption in the infrared portion of the spectrum depends on the frequencies of atomic or molecular vibrations or chemical bonds, and on selection ruleSelection ruleIn physics and chemistry a selection rule, or transition rule, formally constrains the possible transitions of a system from one state to another. Selection rules have been derived for electronic, vibrational, and rotational transitions...
s. Nitrogen and oxygen are not greenhouse gases because the absorption is forbidden by the lack of a molecular dipole moment.
With regard to the scattering of light, the most critical factor is the length scale of any or all of these structural features relative to the wavelength of the light being scattered. Primary material considerations include:
- Crystalline structure: whether or not the atoms or molecules exhibit the long-range order evidenced in crystalline solids.
- Glassy structure: scattering centers include fluctuations in density or composition.
- Microstructure: scattering centers include internal surfaces such as grain boundaries, crystallographic defectCrystallographic defectCrystalline solids exhibit a periodic crystal structure. The positions of atoms or molecules occur on repeating fixed distances, determined by the unit cell parameters. However, the arrangement of atom or molecules in most crystalline materials is not perfect...
s and microscopic pores. - Organic materials: scattering centers include fiber and cell structures and boundaries.
Light scattering in solids
Diffuse reflectionDiffuse reflection
Diffuse reflection is the reflection of light from a surface such that an incident ray is reflected at many angles rather than at just one angle as in the case of specular reflection...
- Generally, when light strikes the surface of a (non-metallic and non-glassy) solid material, it bounces off in all directions due to multiple reflections by the microscopic irregularities inside the material (e.g., the grain boundaries of a polycrystalline
Polycrystalline
Polycrystalline materials are solids that are composed of many crystallites of varying size and orientation. The variation in direction can be random or directed, possibly due to growth and processing conditions. Fiber texture is an example of the latter.Almost all common metals, and many ceramics...
material, or the cell
Cell (biology)
The cell is the basic structural and functional unit of all known living organisms. It is the smallest unit of life that is classified as a living thing, and is often called the building block of life. The Alberts text discusses how the "cellular building blocks" move to shape developing embryos....
or fiber
Fiber
Fiber is a class of materials that are continuous filaments or are in discrete elongated pieces, similar to lengths of thread.They are very important in the biology of both plants and animals, for holding tissues together....
boundaries of an organic material), and by its surface, if it is rough. Diffuse reflection is typically characterized by omni-directional reflection angles. Most of the objects visible to the naked eye are identified via diffuse reflection. Another term commonly used for this type of reflection is “light scattering”. Light scattering from the surfaces of objects is our primary mechanism of physical observation.
Light scattering in liquids and solids depends on the wavelength of the light being scattered. Limits to spatial scales of visibility (using white light) therefore arise, depending on the frequency of the light wave and the physical dimension
Dimension
In physics and mathematics, the dimension of a space or object is informally defined as the minimum number of coordinates needed to specify any point within it. Thus a line has a dimension of one because only one coordinate is needed to specify a point on it...
(or spatial scale) of the scattering center. Visible light has a wavelength scale on the order of a half a micrometer
Micrometer
A micrometer , sometimes known as a micrometer screw gauge, is a device incorporating a calibrated screw used widely for precise measurement of small distances in mechanical engineering and machining as well as most mechanical trades, along with other metrological instruments such as dial, vernier,...
(one millionth of a meter). Scattering centers (or particles) as small as one micrometer have been observed directly in the light microscope
Microscope
A microscope is an instrument used to see objects that are too small for the naked eye. The science of investigating small objects using such an instrument is called microscopy...
(e.g., Brownian motion
Brownian motion
Brownian motion or pedesis is the presumably random drifting of particles suspended in a fluid or the mathematical model used to describe such random movements, which is often called a particle theory.The mathematical model of Brownian motion has several real-world applications...
).
Applications

Grain boundary
A grain boundary is the interface between two grains, or crystallites, in a polycrystalline material. Grain boundaries are defects in the crystal structure, and tend to decrease the electrical and thermal conductivity of the material...
which separate tiny regions of crystalline order. When the size of the scattering center (or grain boundary) is reduced below the size of the wavelength of the light being scattered, the scattering no longer occurs to any significant extent.

Computer modeling of light transmission through translucent ceramic alumina has shown that microscopic pores trapped near grain boundaries act as primary scattering centers. The volume fraction of porosity had to be reduced below 1% for high-quality optical transmission (99.99 percent of theoretical density). This goal has been readily accomplished and amply demonstrated in laboratories and research facilities worldwide using the emerging chemical processing methods encompassed by the methods of sol-gel chemistry and nanotechnology
Nanotechnology
Nanotechnology is the study of manipulating matter on an atomic and molecular scale. Generally, nanotechnology deals with developing materials, devices, or other structures possessing at least one dimension sized from 1 to 100 nanometres...
.

The development of transparent panel products will have other potential advanced applications including high strength, impact-resistant materials that can be used for domestic windows and skylights. Perhaps more important is that walls and other applications will have improved overall strength, especially for high-shear conditions found in high seismic and wind exposures. If the expected improvements in mechanical properties bear out, the traditional limits seen on glazing areas in today's building codes could quickly become outdated if the window area actually contributes to the shear resistance of the wall.
Currently available infrared transparent materials typically exhibit a trade-off between optical performance, mechanical strength and price. For example, sapphire
Sapphire
Sapphire is a gemstone variety of the mineral corundum, an aluminium oxide , when it is a color other than red or dark pink; in which case the gem would instead be called a ruby, considered to be a different gemstone. Trace amounts of other elements such as iron, titanium, or chromium can give...
(crystalline alumina) is very strong, but it is expensive and lacks full transparency throughout the 3–5 micrometer mid-infrared range. Yttria is fully transparent from 3–5 micrometers, but lacks sufficient strength, hardness, and thermal shock resistance for high-performance aerospace applications. Not surprisingly, a combination of these two materials in the form of the yttrium aluminium garnet
Yttrium aluminium garnet
Yttrium aluminium garnet is a synthetic crystalline material of the garnet group. It is also one of three phases of the yttria-aluminium composite, the other two being yttrium aluminium monoclinic and yttrium aluminium perovskite . YAG is commonly used as a host material in various solid-state...
(YAG) is one of the top performers in the field.
Absorption of light in solids
When light strikes an object, it usually has not just a single frequency (or wavelength) but many. Objects have a tendency to selectively absorb, reflect or transmit light of certain frequencies. That is, one object might reflect green light while absorbing all other frequencies of visible light. Another object might selectively transmit blue light while absorbing all other frequencies of visible light. The manner in which visible light interacts with an object is dependent upon the frequency of the light, the nature of the atoms in the object, and often the nature of the electronElectron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s in the atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
s of the object.
Some materials allow much of the light that falls on them to be transmitted through the material without being reflected. Materials that allow the transmission of light waves through them are called optically transparent. Chemically pure (undoped) window glass and clean river or spring water are prime examples of this.
Materials which do not allow the transmission of any light wave frequencies are called opaque
Opacity (optics)
Opacity is the measure of impenetrability to electromagnetic or other kinds of radiation, especially visible light. In radiative transfer, it describes the absorption and scattering of radiation in a medium, such as a plasma, dielectric, shielding material, glass, etc...
. Such substances may have a chemical composition which includes what are referred to as absorption centers. Most materials are composed of materials which are selective in their absorption of light frequencies. Thus they absorb only certain portions of the visible spectrum. The frequencies of the spectrum which are not absorbed are either reflected back or transmitted for our physical observation. In the visible portion of the spectrum, this is what gives rise to color.

- Electronic: Transitions in electron energy levels within the atom (e.g., pigments). These transitions are typically in the ultraviolet (UV) and/or visible portions of the spectrum.
- Vibrational: ResonanceResonanceIn physics, resonance is the tendency of a system to oscillate at a greater amplitude at some frequencies than at others. These are known as the system's resonant frequencies...
in atomic/molecular vibrational modes. These transitions are typically in the infrared portion of the spectrum.
UV-Vis: Electronic transitions
In electronic absorption, the frequency of the incoming light wave is at or near the energy levels of the electrons within the atoms which compose the substance. In this case, the electrons will absorb the energy of the light wave and increase their energy state, often moving outward from the nucleusAtomic nucleus
The nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911, as a result of Ernest Rutherford's interpretation of the famous 1909 Rutherford experiment performed by Hans Geiger and Ernest Marsden, under the direction of Rutherford. The...
of the atom into an outer shell or orbital
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus...
.
The atoms that bind together to make the molecules of any particular substance contain a number of electrons (given by the atomic number
Atomic number
In chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
Z in the periodic chart). Recall that all light waves are electromagnetic in origin. Thus they are affected strongly when coming into contact with negatively charged electrons in matter. When photons (individual packets of light energy) come in contact with the valence electrons of atom, one of several things can and will occur:
- An electron absorbs all of the energy of the photon and re-emits it with different color. This gives rise to luminescenceLuminescenceLuminescence is emission of light by a substance not resulting from heat; it is thus a form of cold body radiation. It can be caused by chemical reactions, electrical energy, subatomic motions, or stress on a crystal. This distinguishes luminescence from incandescence, which is light emitted by a...
, fluorescenceFluorescenceFluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation of a different wavelength. It is a form of luminescence. In most cases, emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation...
and phosphorescencePhosphorescencePhosphorescence is a specific type of photoluminescence related to fluorescence. Unlike fluorescence, a phosphorescent material does not immediately re-emit the radiation it absorbs. The slower time scales of the re-emission are associated with "forbidden" energy state transitions in quantum...
.
- An electron absorbs the energy of the photon and sends it back out the way it came in. This results in reflection or scattering.
- An electron cannot absorb the energy of the photon and the photon continues on its path. This results in transmission (provided no other absorption mechanisms are active).
- An electron selectively absorbs a portion of the photon, and the remaining frequencies are transmitted in the form of spectral color.
Most of the time, it is a combination of the above that happens to the light that hits an object. The electrons in different materials vary in the range of energy that they can absorb. Most glasses, for example, block ultraviolet (UV) light. What happens is the electrons in the glass absorb the energy of the photons in the UV range while ignoring the weaker energy of photons in the visible light spectrum.
Thus, when a material is illuminated, individual photons of light can make the valence electrons of an atom transition to a higher electronic energy level
Energy level
A quantum mechanical system or particle that is bound -- that is, confined spatially—can only take on certain discrete values of energy. This contrasts with classical particles, which can have any energy. These discrete values are called energy levels...
. The photon is destroyed in the process and the absorbed radiant energy is transformed to electric potential energy. Several things can happen then to the absorbed energy. as it may be re-emitted by the electron as radiant energy
Radiant energy
Radiant energy is the energy of electromagnetic waves. The quantity of radiant energy may be calculated by integrating radiant flux with respect to time and, like all forms of energy, its SI unit is the joule. The term is used particularly when radiation is emitted by a source into the...
(in this case the overall effect is in fact a scattering of light), dissipated to the rest of the material (i.e. transformed into heat
Heat
In physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between...
), or the electron can be freed from the atom (as in the photoelectric
Photoelectric effect
In the photoelectric effect, electrons are emitted from matter as a consequence of their absorption of energy from electromagnetic radiation of very short wavelength, such as visible or ultraviolet light. Electrons emitted in this manner may be referred to as photoelectrons...
and Compton effects).
Infrared: Bond stretching
.gif)
Heat
In physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between...
, or thermal energy
Thermal energy
Thermal energy is the part of the total internal energy of a thermodynamic system or sample of matter that results in the system's temperature....
. Thermal energy manifests itself as energy of motion. Thus, heat is motion at the atomic and molecular levels. The primary mode of motion in crystalline substances is vibration
Vibration
Vibration refers to mechanical oscillations about an equilibrium point. The oscillations may be periodic such as the motion of a pendulum or random such as the movement of a tire on a gravel road.Vibration is occasionally "desirable"...
. Any given atom will vibrate around some mean
Mean
In statistics, mean has two related meanings:* the arithmetic mean .* the expected value of a random variable, which is also called the population mean....
or average position within a crystalline structure, surrounded by its nearest neighbors. This vibration in 2-dimensions is equivalent to the oscillation
Oscillation
Oscillation is the repetitive variation, typically in time, of some measure about a central value or between two or more different states. Familiar examples include a swinging pendulum and AC power. The term vibration is sometimes used more narrowly to mean a mechanical oscillation but sometimes...
of a clock’s pendulum. It swings back and forth symmetrically about some mean or average (vertical) position. Atomic and molecular vibrational frequencies may average on the order of 1012 cycles per second (hertz).
When a light wave of a given frequency strikes a material with particles having the same or (resonant) vibrational frequencies, then those particles will absorb the energy of the light wave and transform it into thermal energy of vibrational motion. Since different atoms and molecules have different natural frequencies of vibration, they will selectively absorb different frequencies (or portions of the spectrum) of infrared light. Reflection and transmission of light waves occur because the frequencies of the light waves do not match the natural resonant frequencies of vibration of the objects. When infrared light of these frequencies strikes an object, the energy is reflected or transmitted.
If the object is transparent, then the light waves are passed on to neighboring atoms through the bulk of the material and re-emitted on the opposite side of the object. Such frequencies of light waves are said to be transmitted.
Transparency in insulators
An object may be not transparent either because it reflects the incoming light or because it absorbs the incoming light. Almost all solids reflect a part and absorb a part of the incoming light.When light falls onto a block of metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
, it encounters atoms that are tightly packed in a regular lattice
Lattice model (physics)
In physics, a lattice model is a physical model that is defined on a lattice, as opposed to the continuum of space or spacetime. Lattice models originally occurred in the context of condensed matter physics, where the atoms of a crystal automatically form a lattice. Currently, lattice models are...
and a "sea of electrons" moving randomly between the atoms. In metals, most of these are non-bonding electrons (or free electrons) as opposed to the bonding electrons typically found in covalently bonded or ionically bonded non-metallic (insulating) solids. In a metallic bond, any potential bonding electrons can easily be lost by the atoms in a crystalline structure. The effect of this delocalization is simply to exaggerate the effect of the "sea of electrons". As a result of these electrons, most of the incoming light in metals is reflected back, which is why we see a shiny
Shiny
Shiny is the debut album from Johannesburg-based indie pop band The Bang. The album was released in September 2005. The album's cover art was designed by Daniel Levi, who has directed music videos for Interpol and Massive Attack...
metal surface.
Most insulators (or dielectric
Dielectric
A dielectric is an electrical insulator that can be polarized by an applied electric field. When a dielectric is placed in an electric field, electric charges do not flow through the material, as in a conductor, but only slightly shift from their average equilibrium positions causing dielectric...
materials) are held together by ionic bond
Ionic bond
An ionic bond is a type of chemical bond formed through an electrostatic attraction between two oppositely charged ions. Ionic bonds are formed between a cation, which is usually a metal, and an anion, which is usually a nonmetal. Pure ionic bonding cannot exist: all ionic compounds have some...
s. Thus, these materials do not have free conduction electrons, and the bonding electrons reflect only a small fraction of the incident wave. The remaining frequencies (or wavelengths) are free to propagate (or be transmitted). This class of materials includes all ceramics and glass
Glass
Glass is an amorphous solid material. Glasses are typically brittle and optically transparent.The most familiar type of glass, used for centuries in windows and drinking vessels, is soda-lime glass, composed of about 75% silica plus Na2O, CaO, and several minor additives...
es.
If a dielectric material does not include light-absorbent additive molecules (pigments, dyes, colorants), it is usually transparent to the spectrum of visible light. Color centers (or dye molecules, or "dopants") in a dielectric absorb a portion of the incoming light wave. The remaining frequencies (or wavelengths) are free to be reflected or transmitted. This is how colored glass is produced.
Most liquids and aqueous solutions are highly transparent. For example, water, cooking oil, rubbing alcohol, air, natural gas, are all clear. Absence of structural defects (voids, cracks, etc.) and molecular structure of most liquids are chiefly responsible for their excellent optical transmission. The ability of liquids to "heal" internal defects via viscous flow is one of the reasons why some fibrous materials (e.g., paper or fabric) increase their apparent transparency when wetted. The liquid fills up numerous voids making the material more structurally homogeneous.
Light scattering in an ideal defect-free crystalline (non-metallic) solid which provides no scattering centers for incoming lightwaves will be due primarily to any effects of anharmonicity within the ordered lattice. Lightwave transmission will be highly directional due to the typical anisotropy
Anisotropy
Anisotropy is the property of being directionally dependent, as opposed to isotropy, which implies identical properties in all directions. It can be defined as a difference, when measured along different axes, in a material's physical or mechanical properties An example of anisotropy is the light...
of crystalline substances, which includes their symmetry group
Symmetry group
The symmetry group of an object is the group of all isometries under which it is invariant with composition as the operation...
and Bravais lattice. For example, the seven different crystalline forms of quartz
Quartz
Quartz is the second-most-abundant mineral in the Earth's continental crust, after feldspar. It is made up of a continuous framework of SiO4 silicon–oxygen tetrahedra, with each oxygen being shared between two tetrahedra, giving an overall formula SiO2. There are many different varieties of quartz,...
silica (silicon dioxide
Silicon dioxide
The chemical compound silicon dioxide, also known as silica , is an oxide of silicon with the chemical formula '. It has been known for its hardness since antiquity...
, SiO2) are all clear, transparent materials.
Optical waveguides
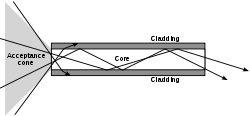

Multi-mode optical fiber
Multi-mode optical fiber is a type of optical fiber mostly used for communication over short distances, such as within a building or on a campus...
) with little or no interference
Adjacent-channel interference
Adjacent-channel interference is interference caused by extraneous power from a signal in an adjacent channel. ACI may be caused by inadequate filtering , improper tuning or poor frequency control .ACI is distinguished from crosstalk.Broadcast...
between competing wavelengths or frequencies. This resonant mode of energy and data transmission via electromagnetic (light) wave propagation is relatively lossless.
An optical fiber is a cylindrical dielectric waveguide that transmits light along its axis by the process of total internal reflection
Total internal reflection
Total internal reflection is an optical phenomenon that happens when a ray of light strikes a medium boundary at an angle larger than a particular critical angle with respect to the normal to the surface. If the refractive index is lower on the other side of the boundary and the incident angle is...
. The fiber consists of a core
Core (optical fiber)
The core of a conventional optical fiber is a cylinder of glass or plastic that runs along the fiber's length. The core is surrounded by a medium with a lower index of refraction, typically a cladding of a different glass, or plastic...
surrounded by a cladding
Cladding (fiber optics)
Cladding is one or more layers of material of lower refractive index, in intimate contact with a core material of higher refractive index. The cladding causes light to be confined to the core of the fiber by total internal reflection at the boundary between the two. Light propagation in the...
layer. To confine the optical signal in the core, the refractive index
Refractive index
In optics the refractive index or index of refraction of a substance or medium is a measure of the speed of light in that medium. It is expressed as a ratio of the speed of light in vacuum relative to that in the considered medium....
of the core must be greater than that of the cladding. The refractive index is the parameter reflecting the speed of light
Speed of light
The speed of light in vacuum, usually denoted by c, is a physical constant important in many areas of physics. Its value is 299,792,458 metres per second, a figure that is exact since the length of the metre is defined from this constant and the international standard for time...
in a material. (Refractive index is the ratio of the speed of light in a vacuum to the speed of light in a given medium. The refractive index of a vacuum is therefore 1). The larger the refractive index, the more slowly light travels in that medium. Typical values for core and cladding of an optical fiber are 1.48 and 1.46, respectively.
When light traveling in a dense medium hits a boundary at a steep angle, the light will be completely reflected. This effect, called total internal reflection
Total internal reflection
Total internal reflection is an optical phenomenon that happens when a ray of light strikes a medium boundary at an angle larger than a particular critical angle with respect to the normal to the surface. If the refractive index is lower on the other side of the boundary and the incident angle is...
, is used in optical fibers to confine light in the core. Light travels along the fiber bouncing back and forth off of the boundary. Because the light must strike the boundary with an angle greater than the critical angle
Total internal reflection
Total internal reflection is an optical phenomenon that happens when a ray of light strikes a medium boundary at an angle larger than a particular critical angle with respect to the normal to the surface. If the refractive index is lower on the other side of the boundary and the incident angle is...
, only light that enters the fiber within a certain range of angles will be propagated. This range of angles is called the acceptance cone of the fiber. The size of this acceptance cone is a function of the refractive index difference between the fiber's core and cladding. Optical waveguides are used as components in integrated optical circuits (e.g. combined with lasers or light-emitting diodes, LEDs) or as the transmission medium in local and long haul optical communication
Optical communication
Optical communication is any form of telecommunication that uses light as the transmission medium.An optical communication system consists of a transmitter, which encodes a message into an optical signal, a channel, which carries the signal to its destination, and a receiver, which reproduces the...
systems.
Mechanisms of attenuation
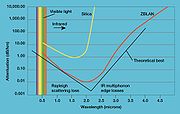
Attenuation
In physics, attenuation is the gradual loss in intensity of any kind of flux through a medium. For instance, sunlight is attenuated by dark glasses, X-rays are attenuated by lead, and light and sound are attenuated by water.In electrical engineering and telecommunications, attenuation affects the...
in fiber optics
Optical fiber
An optical fiber is a flexible, transparent fiber made of a pure glass not much wider than a human hair. It functions as a waveguide, or "light pipe", to transmit light between the two ends of the fiber. The field of applied science and engineering concerned with the design and application of...
, also known as transmission loss, is the reduction in intensity of the light beam (or signal) with respect to distance traveled through a transmission medium. Attenuation coefficients in fiber optics usually use units of dB/km through the medium due to the very high quality of transparency of modern optical transmission media. The medium is usually a fiber of silica glass that confines the incident light beam to the inside. Attenuation is an important factor limiting the transmission of a signal across large distances. In optical fibers the main attenuation source is scattering from molecular level irregularities (Rayleigh scattering
Rayleigh scattering
Rayleigh scattering, named after the British physicist Lord Rayleigh, is the elastic scattering of light or other electromagnetic radiation by particles much smaller than the wavelength of the light. The particles may be individual atoms or molecules. It can occur when light travels through...
) due to structural disorder and compositional fluctuations of the glass structure
Amorphous solid
In condensed matter physics, an amorphous or non-crystalline solid is a solid that lacks the long-range order characteristic of a crystal....
. This same phenomenon is seen as one of the limiting factors in the transparency of infrared missile domes. Further attenuation is caused by light absorbed by residual materials, such as metals or water ions, within the fiber core and inner cladding. Light leakage due to bending, splices, connectors, or other outside forces are other factors resulting in attenuation.
See also
- TurbidityTurbidityTurbidity is the cloudiness or haziness of a fluid caused by individual particles that are generally invisible to the naked eye, similar to smoke in air. The measurement of turbidity is a key test of water quality....
- Brillouin scatteringBrillouin scatteringBrillouin scattering, named after Léon Brillouin, occurs when light in a medium interacts with time dependent optical density variations and changes its energy and path. The density variations may be due to acoustic modes, such as phonons, magnetic modes, such as magnons, or temperature gradients...
- Colloidal crystalColloidal crystalA colloidal crystal is an ordered array of colloid particles, analogous to a standard crystal whose repeating subunits are atoms or molecules. A natural example of this phenomenon can be found in the gem opal, where spheres of silica assume a close-packed locally periodic structure under moderate...
- Light scatteringLight scatteringLight scattering is a form of scattering in which light is the form of propagating energy which is scattered. Light scattering can be thought of as the deflection of a ray from a straight path, for example by irregularities in the propagation medium, particles, or in the interface between two media...
- Optical fiberOptical fiberAn optical fiber is a flexible, transparent fiber made of a pure glass not much wider than a human hair. It functions as a waveguide, or "light pipe", to transmit light between the two ends of the fiber. The field of applied science and engineering concerned with the design and application of...
- Pellicle mirrorPellicle mirrorright|thumb|The pellicle mirror of the [[Canon EOS RT]]A pellicle mirror is an ultra-thin, ultra-lightweight semi-transparent mirror employed in the light path of an optical instrument, splitting the light beam into two separate beams, both of reduced light intensity...
- Photonic crystalPhotonic crystalPhotonic crystals are periodic optical nanostructures that are designed to affect the motion of photons in a similar way that periodicity of a semiconductor crystal affects the motion of electrons...
- Transparent metals
- Transparent ceramics
Further reading
- Electrodynamics of continuous media, Landau, L. D., Lifshits. E.M. and Pitaevskii, L.P., (Pergamon Press, Oxford, 1984)
- Laser Light Scattering: Basic Principles and Practice Chu, B., 2nd Edn. (Academic Press, New York 1992)
- Solid State Laser Engineering, W. Koechner (Springer-Verlag, New York, 1999)
- Introduction to Chemical Physics, J.C. Slater (McGraw-Hill, New York, 1939)
- Modern Theory of Solids, F. Seitz, (McGraw-Hill, New York, 1940)
- Modern Aspects of the Vitreous State, J.D.MacKenzie, Ed. (Butterworths, London, 1960)

