Forms of energy
Encyclopedia

Physical science
Physical science is an encompassing term for the branches of natural science and science that study non-living systems, in contrast to the life sciences...
s, several forms of energy
Energy
In physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
have been defined. These include:
|
Magnetic energy Magnetic energy and electric energy are related by Maxwell's equations. The potential energy of a magnet of magnetic moment m in a magnetic field B is defined as the work of magnetic force on re-alignment of the vector of the magnetic dipole moment, and is equal:while the energy stored in an... Elastic energy Elastic energy is the potential mechanical energy stored in the configuration of a material or physical system as work is performed to distort its volume or shape.... Mechanical energy In physics, mechanical energy is the sum of potential energy and kinetic energy present in the components of a mechanical system. It is the energy associated with the motion and position of an object. The law of conservation of energy states that in an isolated system that is only subject to... Luminous energy In photometry, luminous energy is the perceived energy of light. This is sometimes also called the quantity of light.Luminous energy is not the same as the radiant energy, the corresponding objective physical quantity. This is because the human eye can only see light in the visible spectrum and has... Mass Mass can be defined as a quantitive measure of the resistance an object has to change in its velocity.In physics, mass commonly refers to any of the following three properties of matter, which have been shown experimentally to be equivalent:... |
These energies may be divided into two main groups; kinetic energy
Kinetic energy
The kinetic energy of an object is the energy which it possesses due to its motion.It is defined as the work needed to accelerate a body of a given mass from rest to its stated velocity. Having gained this energy during its acceleration, the body maintains this kinetic energy unless its speed changes...
and potential energy
Potential energy
In physics, potential energy is the energy stored in a body or in a system due to its position in a force field or due to its configuration. The SI unit of measure for energy and work is the Joule...
. Other familiar types of energy are a varying mix of both potential and kinetic energy. Energy may be transformed between these forms.
The above list of the known possible forms of energy is not necessarily complete. Whenever physical scientists discover that a certain phenomenon appears to violate the law of energy conservation
Conservation of energy
The nineteenth century law of conservation of energy is a law of physics. It states that the total amount of energy in an isolated system remains constant over time. The total energy is said to be conserved over time...
, new forms may be added, as is the case with dark energy
Dark energy
In physical cosmology, astronomy and celestial mechanics, dark energy is a hypothetical form of energy that permeates all of space and tends to accelerate the expansion of the universe. Dark energy is the most accepted theory to explain recent observations that the universe appears to be expanding...
, a hypothetical form of energy that permeates all of space and tends to increase the rate of expansion of the universe.
Classical mechanics
Classical mechanics
In physics, classical mechanics is one of the two major sub-fields of mechanics, which is concerned with the set of physical laws describing the motion of bodies under the action of a system of forces...
distinguishes between potential energy
Potential energy
In physics, potential energy is the energy stored in a body or in a system due to its position in a force field or due to its configuration. The SI unit of measure for energy and work is the Joule...
, which is a function of the position of an object, and kinetic energy
Kinetic energy
The kinetic energy of an object is the energy which it possesses due to its motion.It is defined as the work needed to accelerate a body of a given mass from rest to its stated velocity. Having gained this energy during its acceleration, the body maintains this kinetic energy unless its speed changes...
, which is a function of its movement
Motion (physics)
In physics, motion is a change in position of an object with respect to time. Change in action is the result of an unbalanced force. Motion is typically described in terms of velocity, acceleration, displacement and time . An object's velocity cannot change unless it is acted upon by a force, as...
. Both position and movement are relative to a frame of reference
Frame of reference
A frame of reference in physics, may refer to a coordinate system or set of axes within which to measure the position, orientation, and other properties of objects in it, or it may refer to an observational reference frame tied to the state of motion of an observer.It may also refer to both an...
, which must be specified: this is often (and originally) an arbitrary fixed point on the surface of the Earth, the terrestrial frame of reference. It has been attempted to categorize all forms of energy as either kinetic or potential: this is not incorrect, but neither is it clear that it is a real simplification, as Feynman points out:
| Mechanical energy is converted | |
|---|---|
| into | by |
| Mechanical energy Mechanical energy In physics, mechanical energy is the sum of potential energy and kinetic energy present in the components of a mechanical system. It is the energy associated with the motion and position of an object. The law of conservation of energy states that in an isolated system that is only subject to... |
Lever Lever In physics, a lever is a rigid object that is used with an appropriate fulcrum or pivot point to either multiply the mechanical force that can be applied to another object or resistance force , or multiply the distance and speed at which the opposite end of the rigid object travels.This leverage... |
| Thermal energy Thermal energy Thermal energy is the part of the total internal energy of a thermodynamic system or sample of matter that results in the system's temperature.... |
Brake Brake A brake is a mechanical device which inhibits motion. Its opposite component is a clutch. The rest of this article is dedicated to various types of vehicular brakes.... s |
| Electric energy | Dynamo Dynamo - Engineering :* Dynamo, a magnetic device originally used as an electric generator* Dynamo theory, a theory relating to magnetic fields of celestial bodies* Solar dynamo, the physical process that generates the Sun's magnetic field- Software :... |
| Electromagnetic radiation Electromagnetic radiation Electromagnetic radiation is a form of energy that exhibits wave-like behavior as it travels through space... |
Synchrotron Synchrotron A synchrotron is a particular type of cyclic particle accelerator in which the magnetic field and the electric field are carefully synchronised with the travelling particle beam. The proton synchrotron was originally conceived by Sir Marcus Oliphant... |
| Chemical energy Chemical energy Chemical energy is the potential of a chemical substance to undergo a transformation through a chemical reaction or, to transform other chemical substances... |
Match Match A match is a tool for starting a fire under controlled conditions. A typical modern match is made of a small wooden stick or stiff paper. One end is coated with a material that can be ignited by frictional heat generated by striking the match against a suitable surface... es |
| Nuclear energy Nuclear binding energy Nuclear binding energy is the energy required to split a nucleus of an atom into its component parts. The component parts are neutrons and protons, which are collectively called nucleons... |
Particle accelerator Particle accelerator A particle accelerator is a device that uses electromagnetic fields to propel charged particles to high speeds and to contain them in well-defined beams. An ordinary CRT television set is a simple form of accelerator. There are two basic types: electrostatic and oscillating field accelerators.In... |
Mechanical energy
General non-relativistic mechanicsMechanical energy (symbols EM or E) manifest in many forms, but can be broadly classified into potential energy (Ep, V, U or Φ) and kinetic energy (Ek or T). The term potential energy is a very general term, because it exists in all force fields, such as gravitation, electrostatic and magnetic fields. Potential energy refers to the energy any object gains due to its position in a force field.
The relatation between mechanical energy with kinetic and potential energy is simply
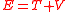 .
.Lagrangian and Hamiltionian mechanics
In more advanced topics, kinetic plus potential energy is physically the total energy of the system, but also known as the Hamiltonian of the system:
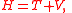
used in Hamilton's equations of motion, to obtain equations describing a classical system in terms of energy rather than forces. The Hamiltonian is just a mathematical expression, rather than a form of energy.
Another analogous quantity of profound applicability and efficiency is the Lagrangian of the system:
 ,
,used in Lagrange's equations of motion, which serve the same peupose as Hamilton's equations.
Mechanical work
Translational motionIf F is the force and r is the displacement
Displacement (vector)
A displacement is the shortest distance from the initial to the final position of a point P. Thus, it is the length of an imaginary straight path, typically distinct from the path actually travelled by P...
, then the change in mechanical work done along the path between positions r1 and r2 due to the force is, in integral form:
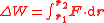 ,
,(the dot represents the scalar product of the two vectors). The general equation above can be simplified in a number of common cases, notably when dealing with gravity or with elastic forces. If the force is conservative the equation can be written in differential form as
 .
.Rotational motion
The rotational analogue is the work done by a torque
Torque
Torque, moment or moment of force , is the tendency of a force to rotate an object about an axis, fulcrum, or pivot. Just as a force is a push or a pull, a torque can be thought of as a twist....
τ, between the angles θ1 and θ2,
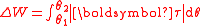 .
.Potential energy
Potential energy is defined as the work done against a given force in changing the position of an object with respect to a reference position, often taken to be infinite separation. In other words it is the work done on the object to give it that much energy. Changes in work and potential energy are related simply,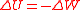 .
.The name "potential" energy originally signified the idea that the energy could readily be transferred as work — at least in an idealized system (reversible process, see below). This is not completely true for any real system, but is often a reasonable first approximation in classical mechanics.
Elastic potential energy

Elastic potential energy is defined as a work needed to compress or extend a spring. The tension/compression force F in a spring or any other system which obeys Hooke's law
Hooke's law
In mechanics, and physics, Hooke's law of elasticity is an approximation that states that the extension of a spring is in direct proportion with the load applied to it. Many materials obey this law as long as the load does not exceed the material's elastic limit. Materials for which Hooke's law...
is proportional to the extension/compression x,
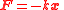 ,
,where k is the force constant of the particular spring or system. In this case the force is conservative, the calculated work becomes
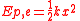 .
.If k is not constant the above equation will fail. Hooke's law is a good approximation for behaviour of chemical bond
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
s under stable conditions, i.e. when they are not being broken or formed.
Kinetic energy
General scopeKinetic energy is the work required to accelerate an object to a given speed. These values are expressed as:
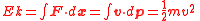
Special Relativistic mechanics
At speeds approac hing the speed of light
Speed of light
The speed of light in vacuum, usually denoted by c, is a physical constant important in many areas of physics. Its value is 299,792,458 metres per second, a figure that is exact since the length of the metre is defined from this constant and the international standard for time...
, c, this work must be calculated using Lorentz transformations, and applying mass and energy conservation, which results in the following:

where
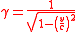 is the lorentz factor
is the lorentz factorLorentz factor
The Lorentz factor or Lorentz term appears in several equations in special relativity, including time dilation, length contraction, and the relativistic mass formula. Because of its ubiquity, physicists generally represent it with the shorthand symbol γ . It gets its name from its earlier...
.
Here the two terms on the right hand side are identified with the total energy and the rest energy of the object, respectively. This equation reduces to the one above it, at small (compared to c) speed. The kinetic energy is zero at v=0 (when γ = 1), so that at rest, the total energy is the rest energy. So a mass at rest in some inertial reference frame has a corresponding amount of rest energy equal to:

All masses at rest have a tremendous amuont of energy, due to the proportionality factor of c2.
Surface energy
If there is any kind of tension in a surface, such as a stretched sheet of rubber or material interfaces, it is possible to define surface energy.If γ is the surface tension, and S = surface area, then the work done W to increase the area by a unit area is the surface energy:
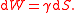
In particular, any meeting of dissimilar materials that do not mix will result in some kind of surface tension
Surface tension
Surface tension is a property of the surface of a liquid that allows it to resist an external force. It is revealed, for example, in floating of some objects on the surface of water, even though they are denser than water, and in the ability of some insects to run on the water surface...
, if there is freedom for the surfaces to move then, as seen in capillary surface
Capillary surface
In fluid mechanics and mathematics, a capillary surface is a surface that represents the interface between two different fluids. As a consequence of being a surface, a capillary surface has no thickness in slight contrast with most real fluid interfaces....
s for example, the minimum energy will as usual be sought.
A minimal surface
Minimal surface
In mathematics, a minimal surface is a surface with a mean curvature of zero.These include, but are not limited to, surfaces of minimum area subject to various constraints....
, for example, represents the smallest possible energy that a surface can have if its energy is proportional to the area of the surface. For this reason, (open) soap films of small size are minimal surfaces (small size reduces gravity effects, and openness prevents pressure from building up. Note that a bubble is a minimum energy surface but not a minimal surface
Minimal surface
In mathematics, a minimal surface is a surface with a mean curvature of zero.These include, but are not limited to, surfaces of minimum area subject to various constraints....
by definition).
Sound energy
Sound is a form of mechanical vibration which propagates through any mechanical medium. It is closely related to the ability of the human ear to perceive sound. The wide outer area of the ear is maximized to collect sound vibrations. It is amplified and passed through the outer ear, striking the eardrum, which transmits sounds into the inner ear. Auditory nerves fire according to the particular vibrations of the sound waves in the inner ear, which designate such things as the pitch and volume of the sound. The ear is set up in an optimal way to interpret sound energy in the form of vibrations.Gravitational potential energy
The gravitational force very near the surface of a massive body (e.g. a planet) varies very little with small changes in height, h, and locally is equal mg where m is massMass
Mass can be defined as a quantitive measure of the resistance an object has to change in its velocity.In physics, mass commonly refers to any of the following three properties of matter, which have been shown experimentally to be equivalent:...
, and g is the gravitational acceleration
Gravitational acceleration
In physics, gravitational acceleration is the acceleration on an object caused by gravity. Neglecting friction such as air resistance, all small bodies accelerate in a gravitational field at the same rate relative to the center of mass....
(AKA field strength). At the Earth's surface g = 9.81 m s-1. In these cases, the gravitational potential energy is given by

A more general expression for the potential energy due to Newtonian gravitation between two bodies of masses m1 and m2, is
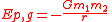 ,
,where r is the separation between the two bodies and G is the gravitational constant
Gravitational constant
The gravitational constant, denoted G, is an empirical physical constant involved in the calculation of the gravitational attraction between objects with mass. It appears in Newton's law of universal gravitation and in Einstein's theory of general relativity. It is also known as the universal...
,
6.6742(10) × 10-11 m3 kg-1 s-2. In this case, the zero potential reference point is the infinite separation of the two bodies. Care must be taken that these masses are point masses or uniform spherical solids/shells. It cannot be applied directly to any objects of any shape and any mass.
In terms of the gravitational potential (Φ, U or V), the potential energy is (by definition of gravitational potential),
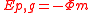 .
.Thermal energy
| Thermal energy is converted | |
|---|---|
| into | by |
| Mechanical energy Mechanical energy In physics, mechanical energy is the sum of potential energy and kinetic energy present in the components of a mechanical system. It is the energy associated with the motion and position of an object. The law of conservation of energy states that in an isolated system that is only subject to... |
Steam turbine Steam turbine A steam turbine is a mechanical device that extracts thermal energy from pressurized steam, and converts it into rotary motion. Its modern manifestation was invented by Sir Charles Parsons in 1884.... |
| Thermal energy Thermal energy Thermal energy is the part of the total internal energy of a thermodynamic system or sample of matter that results in the system's temperature.... |
Heat exchanger Heat exchanger A heat exchanger is a piece of equipment built for efficient heat transfer from one medium to another. The media may be separated by a solid wall, so that they never mix, or they may be in direct contact... |
| Electric energy | Thermocouple Thermocouple A thermocouple is a device consisting of two different conductors that produce a voltage proportional to a temperature difference between either end of the pair of conductors. Thermocouples are a widely used type of temperature sensor for measurement and control and can also be used to convert a... |
| Electromagnetic radiation Electromagnetic radiation Electromagnetic radiation is a form of energy that exhibits wave-like behavior as it travels through space... |
Hot objects Black body A black body is an idealized physical body that absorbs all incident electromagnetic radiation. Because of this perfect absorptivity at all wavelengths, a black body is also the best possible emitter of thermal radiation, which it radiates incandescently in a characteristic, continuous spectrum... |
| Chemical energy Chemical energy Chemical energy is the potential of a chemical substance to undergo a transformation through a chemical reaction or, to transform other chemical substances... |
Blast furnace Blast furnace A blast furnace is a type of metallurgical furnace used for smelting to produce industrial metals, generally iron.In a blast furnace, fuel and ore and flux are continuously supplied through the top of the furnace, while air is blown into the bottom of the chamber, so that the chemical reactions... |
| Nuclear energy Nuclear binding energy Nuclear binding energy is the energy required to split a nucleus of an atom into its component parts. The component parts are neutrons and protons, which are collectively called nucleons... |
Supernova Supernova A supernova is a stellar explosion that is more energetic than a nova. It is pronounced with the plural supernovae or supernovas. Supernovae are extremely luminous and cause a burst of radiation that often briefly outshines an entire galaxy, before fading from view over several weeks or months... |
General scope
Thermal energy (of some state of matter - gas, plasma, solid, etc.) is the energy associated with the microscopical random motion of particles constituting the media. For example, in case of monoatomic gas it is just a kinetic energy of motion of atoms of gas as measured in the reference frame of the center of mass of gas. In case of molecules in the gas rotational and vibrational energy is involved. In the case of liquids and solids there is also potential energy (of interaction of atoms) involved, and so on.
A heat is defined as a transfer (flow) of thermal energy across certain boundary (for example, from a hot body to cold via the area of their contact. A practical definition for small transfers of heat is
where Cv is the heat capacity
Heat capacity
Heat capacity , or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a substance's temperature by a given amount...
of the system. This definition will fail if the system undergoes a phase transition
Phase transition
A phase transition is the transformation of a thermodynamic system from one phase or state of matter to another.A phase of a thermodynamic system and the states of matter have uniform physical properties....
—e.g. if ice is melting to water—as in these cases the system can absorb heat without increasing its temperature. In more complex systems, it is preferable to use the concept of internal energy
Internal energy
In thermodynamics, the internal energy is the total energy contained by a thermodynamic system. It is the energy needed to create the system, but excludes the energy to displace the system's surroundings, any energy associated with a move as a whole, or due to external force fields. Internal...
rather than that of thermal energy (see Chemical energy below).
Despite the theoretical problems, the above definition is useful in the experimental measurement of energy changes. In a wide variety of situations, it is possible to use the energy released by a system to raise the temperature of another object, e.g. a bath of water. It is also possible to measure the amount of electric energy required to raise the temperature of the object by the same amount. The calorie
Calorie
The calorie is a pre-SI metric unit of energy. It was first defined by Nicolas Clément in 1824 as a unit of heat, entering French and English dictionaries between 1841 and 1867. In most fields its use is archaic, having been replaced by the SI unit of energy, the joule...
was originally defined as the amount of energy required to raise the temperature of one gram of water by 1 °C (approximately 4.1855 J, although the definition later changed), and the British thermal unit
British thermal unit
The British thermal unit is a traditional unit of energy equal to about 1055 joules. It is approximately the amount of energy needed to heat of water, which is exactly one tenth of a UK gallon or about 0.1198 US gallons, from 39°F to 40°F...
was defined as the energy required to heat one pound
Pound (mass)
The pound or pound-mass is a unit of mass used in the Imperial, United States customary and other systems of measurement...
of water by 1 °F (later fixed as 1055.06 J).
Kinetic theory
In kinetic theory
Kinetic theory
The kinetic theory of gases describes a gas as a large number of small particles , all of which are in constant, random motion. The rapidly moving particles constantly collide with each other and with the walls of the container...
which describes the ideal gas
Ideal gas
An ideal gas is a theoretical gas composed of a set of randomly-moving, non-interacting point particles. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics.At normal conditions such as...
, the thermal energy per degree of freedom
Degrees of freedom (physics and chemistry)
A degree of freedom is an independent physical parameter, often called a dimension, in the formal description of the state of a physical system...
is given by:
where df is the number of degrees of freedom and ksub>B is the Boltzmann constant. The total themeral energies would equal the total internal energy of the gas, since intermolecular potential energy is neglected in this theory. The term kBT occurs very frequently into statistical thermodynamics.
Electric energy
| Electric energy is converted | |
|---|---|
| into | by |
| Mechanical energy Mechanical energy In physics, mechanical energy is the sum of potential energy and kinetic energy present in the components of a mechanical system. It is the energy associated with the motion and position of an object. The law of conservation of energy states that in an isolated system that is only subject to... |
Electric motor Electric motor An electric motor converts electrical energy into mechanical energy.Most electric motors operate through the interaction of magnetic fields and current-carrying conductors to generate force... |
| Thermal energy Thermal energy Thermal energy is the part of the total internal energy of a thermodynamic system or sample of matter that results in the system's temperature.... |
Resistor Resistor A linear resistor is a linear, passive two-terminal electrical component that implements electrical resistance as a circuit element.The current through a resistor is in direct proportion to the voltage across the resistor's terminals. Thus, the ratio of the voltage applied across a resistor's... |
| Electric energy | Transformer Transformer A transformer is a device that transfers electrical energy from one circuit to another through inductively coupled conductors—the transformer's coils. A varying current in the first or primary winding creates a varying magnetic flux in the transformer's core and thus a varying magnetic field... |
| Electromagnetic radiation Electromagnetic radiation Electromagnetic radiation is a form of energy that exhibits wave-like behavior as it travels through space... |
Light-emitting diode Light-emitting diode A light-emitting diode is a semiconductor light source. LEDs are used as indicator lamps in many devices and are increasingly used for other lighting... |
| Chemical energy Chemical energy Chemical energy is the potential of a chemical substance to undergo a transformation through a chemical reaction or, to transform other chemical substances... |
Electrolysis Electrolysis In chemistry and manufacturing, electrolysis is a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction... |
| Nuclear energy Nuclear binding energy Nuclear binding energy is the energy required to split a nucleus of an atom into its component parts. The component parts are neutrons and protons, which are collectively called nucleons... |
Synchrotron Synchrotron A synchrotron is a particular type of cyclic particle accelerator in which the magnetic field and the electric field are carefully synchronised with the travelling particle beam. The proton synchrotron was originally conceived by Sir Marcus Oliphant... |
Electrostatic energy
General scopeThe electric potential energy
Electric potential energy
Electric potential energy, or electrostatic potential energy, is a potential energy that results from conservative Coulomb forces and is associated with the configuration of a particular set of point charges within a defined system...
of given configuration of charges is defined as the work
Work (thermodynamics)
In thermodynamics, work performed by a system is the energy transferred to another system that is measured by the external generalized mechanical constraints on the system. As such, thermodynamic work is a generalization of the concept of mechanical work in mechanics. Thermodynamic work encompasses...
which must be done against the Coulomb force to rearrange charges from infinite separation to this configuration (or the work done by the Coulomb force separating the charges from this configuration to infinity). For two point-like charges Q1 and Q2 at a distance r this work, and hence electric potential energy is equal to:
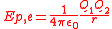
where ε0 is the electric constant
Electric constant
The physical constant ε0, commonly called the vacuum permittivity, permittivity of free space or electric constant is an ideal, physical constant, which is the value of the absolute dielectric permittivity of classical vacuum...
of a vacuum, 107/4πc02 or 8.854188… × 10−12 F m−1. In terms of electrostatic potential (ϕ for absolute, V for differance in potential), again by definition, electrostatic potential energy is given by:
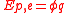 .
.If the charge is accumulated in a capacitor
Capacitor
A capacitor is a passive two-terminal electrical component used to store energy in an electric field. The forms of practical capacitors vary widely, but all contain at least two electrical conductors separated by a dielectric ; for example, one common construction consists of metal foils separated...
(of capacitance
Capacitance
In electromagnetism and electronics, capacitance is the ability of a capacitor to store energy in an electric field. Capacitance is also a measure of the amount of electric potential energy stored for a given electric potential. A common form of energy storage device is a parallel-plate capacitor...
C), the reference configuration is usually selected not to be infinite separation of charges, but vice versa - charges at an extremely close proximity to each other (so there is zero net charge on each plate of a capacitor). The justification for this choice is purely practical - it is easier to measure both voltage difference and magnitude of charges on a capacitor plates not versus infinite separation of charges but rather versus discharged capacitor where charges return to close proximity to each other (electrons and ions recombine making the plates neutral). In this case the work and thus the electric potential energy becomes
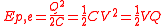 ,
,(different forms obtained using the definition of capacitance).
Electric energy
Electric circuitsIf an electric current
Electric current
Electric current is a flow of electric charge through a medium.This charge is typically carried by moving electrons in a conductor such as wire...
passes through a resistor
Resistor
A linear resistor is a linear, passive two-terminal electrical component that implements electrical resistance as a circuit element.The current through a resistor is in direct proportion to the voltage across the resistor's terminals. Thus, the ratio of the voltage applied across a resistor's...
, electric energy is converted to heat; if the current passes through an electric appliance, some of the electric energy will be converted into other forms of energy (although some will always be lost as heat). The amount of electric energy due to an electric current can be expressed in a number of different ways:
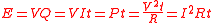
where V is the electric potential difference (in volt
Volt
The volt is the SI derived unit for electric potential, electric potential difference, and electromotive force. The volt is named in honor of the Italian physicist Alessandro Volta , who invented the voltaic pile, possibly the first chemical battery.- Definition :A single volt is defined as the...
s), Q is the charge (in coulombs), I is the current (in ampere
Ampere
The ampere , often shortened to amp, is the SI unit of electric current and is one of the seven SI base units. It is named after André-Marie Ampère , French mathematician and physicist, considered the father of electrodynamics...
s), t is the time for which the current flows (in seconds), P is the power
Power (physics)
In physics, power is the rate at which energy is transferred, used, or transformed. For example, the rate at which a light bulb transforms electrical energy into heat and light is measured in watts—the more wattage, the more power, or equivalently the more electrical energy is used per unit...
(in watt
Watt
The watt is a derived unit of power in the International System of Units , named after the Scottish engineer James Watt . The unit, defined as one joule per second, measures the rate of energy conversion.-Definition:...
s) and R is the electric resistance (in ohm
Ohm
The ohm is the SI unit of electrical resistance, named after German physicist Georg Simon Ohm.- Definition :The ohm is defined as a resistance between two points of a conductor when a constant potential difference of 1 volt, applied to these points, produces in the conductor a current of 1 ampere,...
s). The last of these expressions is important in the practical measurement of energy, as potential difference, resistance and time can all be measured with considerable accuracy.
Magnetic energy
General scopeThere is no fundamental difference between magnetic energy and electric energy: the two phenomena are related by Maxwell's equations
Maxwell's equations
Maxwell's equations are a set of partial differential equations that, together with the Lorentz force law, form the foundation of classical electrodynamics, classical optics, and electric circuits. These fields in turn underlie modern electrical and communications technologies.Maxwell's equations...
. The potential energy of a magnet
Magnet
A magnet is a material or object that produces a magnetic field. This magnetic field is invisible but is responsible for the most notable property of a magnet: a force that pulls on other ferromagnetic materials, such as iron, and attracts or repels other magnets.A permanent magnet is an object...
of magnetic moment
Magnetic moment
The magnetic moment of a magnet is a quantity that determines the force that the magnet can exert on electric currents and the torque that a magnetic field will exert on it...
m in a magnetic field
Magnetic field
A magnetic field is a mathematical description of the magnetic influence of electric currents and magnetic materials. The magnetic field at any given point is specified by both a direction and a magnitude ; as such it is a vector field.Technically, a magnetic field is a pseudo vector;...
B is defined as the work
Mechanical work
In physics, work is a scalar quantity that can be described as the product of a force times the distance through which it acts, and it is called the work of the force. Only the component of a force in the direction of the movement of its point of application does work...
of magnetic force (actually of magnetic torque
Torque
Torque, moment or moment of force , is the tendency of a force to rotate an object about an axis, fulcrum, or pivot. Just as a force is a push or a pull, a torque can be thought of as a twist....
) on re-alignment of the vector of the magnetic dipole moment, and is equal to:
 .
.Electric circuits
The energy stored in an inductor
Inductor
An inductor is a passive two-terminal electrical component used to store energy in a magnetic field. An inductor's ability to store magnetic energy is measured by its inductance, in units of henries...
(of inductance
Inductance
In electromagnetism and electronics, inductance is the ability of an inductor to store energy in a magnetic field. Inductors generate an opposing voltage proportional to the rate of change in current in a circuit...
L) carrying current I is
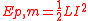 .
.This second expression forms the basis for superconducting magnetic energy storage
Superconducting magnetic energy storage
Superconducting Magnetic Energy Storage systems store energy in the magnetic field created by the flow of direct current in a superconducting coil which has been cryogenically cooled to a temperature below its superconducting critical temperature....
.
Electromagnetic energy
| Electromagnetic radiation is converted | |
|---|---|
| into | by |
| Mechanical energy Mechanical energy In physics, mechanical energy is the sum of potential energy and kinetic energy present in the components of a mechanical system. It is the energy associated with the motion and position of an object. The law of conservation of energy states that in an isolated system that is only subject to... |
Solar sail Solar sail Solar sails are a form of spacecraft propulsion using the radiation pressure of light from a star or laser to push enormous ultra-thin mirrors to high speeds.... |
| Thermal energy Thermal energy Thermal energy is the part of the total internal energy of a thermodynamic system or sample of matter that results in the system's temperature.... |
Solar collector Solar collector -See also:*Solar thermal collector*Solar water heating*Solar air heating*Photovoltaic module*Renewable heat*Concentrating solar power... |
| Electric energy | Solar cell Solar cell A solar cell is a solid state electrical device that converts the energy of light directly into electricity by the photovoltaic effect.... |
| Electromagnetic radiation Electromagnetic radiation Electromagnetic radiation is a form of energy that exhibits wave-like behavior as it travels through space... |
Non-linear optics |
| Chemical energy Chemical energy Chemical energy is the potential of a chemical substance to undergo a transformation through a chemical reaction or, to transform other chemical substances... |
Photosynthesis Photosynthesis Photosynthesis is a chemical process that converts carbon dioxide into organic compounds, especially sugars, using the energy from sunlight. Photosynthesis occurs in plants, algae, and many species of bacteria, but not in archaea. Photosynthetic organisms are called photoautotrophs, since they can... |
| Nuclear energy Nuclear binding energy Nuclear binding energy is the energy required to split a nucleus of an atom into its component parts. The component parts are neutrons and protons, which are collectively called nucleons... |
Mössbauer spectroscopy |
Calculating work
Mechanical work
In physics, work is a scalar quantity that can be described as the product of a force times the distance through which it acts, and it is called the work of the force. Only the component of a force in the direction of the movement of its point of application does work...
needed to create an electric or magnetic field in unit volume (say, in a capacitor or an inductor) results in the electric and magnetic fields energy densities
Energy density
Energy density is a term used for the amount of energy stored in a given system or region of space per unit volume. Often only the useful or extractable energy is quantified, which is to say that chemically inaccessible energy such as rest mass energy is ignored...
:
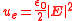 and
and 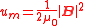 ,
,in SI units.
Electromagnetic radiation, such as microwave
Microwave
Microwaves, a subset of radio waves, have wavelengths ranging from as long as one meter to as short as one millimeter, or equivalently, with frequencies between 300 MHz and 300 GHz. This broad definition includes both UHF and EHF , and various sources use different boundaries...
s, visible light or gamma ray
Gamma ray
Gamma radiation, also known as gamma rays or hyphenated as gamma-rays and denoted as γ, is electromagnetic radiation of high frequency . Gamma rays are usually naturally produced on Earth by decay of high energy states in atomic nuclei...
s, represents a flow of electromagnetic energy. Applying the above expressions to magnetic and electric components of electromagnetic field both the volumetric density and the flow of energy in EM field can be calculated. The resulting Poynting vector
Poynting vector
In physics, the Poynting vector can be thought of as representing the directional energy flux density of an electromagnetic field. It is named after its inventor John Henry Poynting. Oliver Heaviside and Nikolay Umov independently co-invented the Poynting vector...
, which is expressed as
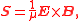
in SI units, gives the density of the flow of energy and its direction.
The energy of electromagnetic radiation is quantized (has discrete energy levels). The energy of a photon is:
 ,
,so the spacing between energy levels is:
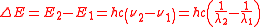 ,
,where h is the Planck constant
Planck constant
The Planck constant , also called Planck's constant, is a physical constant reflecting the sizes of energy quanta in quantum mechanics. It is named after Max Planck, one of the founders of quantum theory, who discovered it in 1899...
, 6.6260693(11)×10−34 Js, and ν is the frequency
Frequency
Frequency is the number of occurrences of a repeating event per unit time. It is also referred to as temporal frequency.The period is the duration of one cycle in a repeating event, so the period is the reciprocal of the frequency...
of the radiation. This quantity of electromagnetic energy is usually called a photon. The photons which make up visible light have energies of 270–520 yJ, equivalent to 160–310 kJ/mol, the strength of weaker chemical bond
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
s.
Chemical energy
| Chemical energy is converted | |
|---|---|
| into | by |
| Mechanical energy Mechanical energy In physics, mechanical energy is the sum of potential energy and kinetic energy present in the components of a mechanical system. It is the energy associated with the motion and position of an object. The law of conservation of energy states that in an isolated system that is only subject to... |
Muscle Muscle Muscle is a contractile tissue of animals and is derived from the mesodermal layer of embryonic germ cells. Muscle cells contain contractile filaments that move past each other and change the size of the cell. They are classified as skeletal, cardiac, or smooth muscles. Their function is to... |
| Thermal energy Thermal energy Thermal energy is the part of the total internal energy of a thermodynamic system or sample of matter that results in the system's temperature.... |
Fire Fire Fire is the rapid oxidation of a material in the chemical process of combustion, releasing heat, light, and various reaction products. Slower oxidative processes like rusting or digestion are not included by this definition.... |
| Electric energy | Fuel cell Fuel cell A fuel cell is a device that converts the chemical energy from a fuel into electricity through a chemical reaction with oxygen or another oxidizing agent. Hydrogen is the most common fuel, but hydrocarbons such as natural gas and alcohols like methanol are sometimes used... |
| Electromagnetic radiation Electromagnetic radiation Electromagnetic radiation is a form of energy that exhibits wave-like behavior as it travels through space... |
Glowworm Glowworm Glowworm, or glow worm, is the common name for various groups of insect larvae and adult larviform females that glow through bioluminescence. They may sometimes resemble worms, but all are insects .-Classification:Major families are:* Lampyridae , found around the world... s |
| Chemical energy Chemical energy Chemical energy is the potential of a chemical substance to undergo a transformation through a chemical reaction or, to transform other chemical substances... |
Chemical reaction Chemical reaction A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity... |
Chemical energy
Chemical energy
Chemical energy is the potential of a chemical substance to undergo a transformation through a chemical reaction or, to transform other chemical substances...
is the energy due to associations of atoms in molecules and various other kinds of aggregates of matter
Matter
Matter is a general term for the substance of which all physical objects consist. Typically, matter includes atoms and other particles which have mass. A common way of defining matter is as anything that has mass and occupies volume...
. It may be defined as a work done by electric forces during re-arrangement of mutual positions of electric charges, electrons and protons, in the process of aggregation. So, basically it is electrostatic potential energy of electric charges. If the chemical energy of a system decreases during a chemical reaction, the difference is transferred to the surroundings in some form (often heat
Heat
In physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between...
or light
Light
Light or visible light is electromagnetic radiation that is visible to the human eye, and is responsible for the sense of sight. Visible light has wavelength in a range from about 380 nanometres to about 740 nm, with a frequency range of about 405 THz to 790 THz...
); on the other hand if the chemical energy of a system increases as a result of a chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
- the difference then is supplied by the surroundings (usually again in form of heat
Heat
In physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between...
or light
Light
Light or visible light is electromagnetic radiation that is visible to the human eye, and is responsible for the sense of sight. Visible light has wavelength in a range from about 380 nanometres to about 740 nm, with a frequency range of about 405 THz to 790 THz...
). For example,
- when two hydrogenHydrogenHydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
atoms react to form a dihydrogen molecule, the chemical energy decreases by 724 zJ (the bond energyBond energyIn chemistry, bond energy is the measure of bond strength in a chemical bond. It is the heat required to break one Mole of molecules into their individual atoms. For example, the carbon-hydrogen bond energy in methane E is the enthalpy change involved with breaking up one molecule of methane into...
of the H–H bond); - when the electron is completely removed from a hydrogen atom, forming a hydrogen ion (in the gas phase), the chemical energy increases by 2.18 aJ (the ionization energyIonization energyThe ionization energy of a chemical species, i.e. an atom or molecule, is the energy required to remove an electron from the species to a practically infinite distance. Large atoms or molecules have a low ionization energy, while small molecules tend to have higher ionization energies.The property...
of hydrogen).
It is common to quote the changes in chemical energy for one mole
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
of the substance in question: typical values for the change in molar chemical energy during a chemical reaction range from tens to hundreds of kilojoules per mole.
The chemical energy as defined above is also referred to by chemists as the internal energy
Internal energy
In thermodynamics, the internal energy is the total energy contained by a thermodynamic system. It is the energy needed to create the system, but excludes the energy to displace the system's surroundings, any energy associated with a move as a whole, or due to external force fields. Internal...
, U: technically, this is measured by keeping the volume
Volume
Volume is the quantity of three-dimensional space enclosed by some closed boundary, for example, the space that a substance or shape occupies or contains....
of the system constant. Most practical chemistry is performed at constant pressure and, if the volume changes during the reaction (e.g. a gas is given off), a correction must be applied to take account of the work done by or on the atmosphere to obtain the enthalpy
Enthalpy
Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
, H, this correction is the work done by an expanding gas,
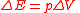 ,
,so the enthalpy now reads;
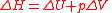 .
.A second correction, for the change in entropy
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
, S, must also be performed to determine whether a chemical reaction will take place or not, giving the Gibbs free energy
Gibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
, G. The correction is the energy required to create order from disorder,
 ,
,so we have;
 .
.These corrections are sometimes negligible, but often not (especially in reactions involving gases).
Since the industrial revolution
Industrial Revolution
The Industrial Revolution was a period from the 18th to the 19th century where major changes in agriculture, manufacturing, mining, transportation, and technology had a profound effect on the social, economic and cultural conditions of the times...
, the burning
Combustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
of coal
Coal
Coal is a combustible black or brownish-black sedimentary rock usually occurring in rock strata in layers or veins called coal beds or coal seams. The harder forms, such as anthracite coal, can be regarded as metamorphic rock because of later exposure to elevated temperature and pressure...
, oil
Petroleum
Petroleum or crude oil is a naturally occurring, flammable liquid consisting of a complex mixture of hydrocarbons of various molecular weights and other liquid organic compounds, that are found in geologic formations beneath the Earth's surface. Petroleum is recovered mostly through oil drilling...
, natural gas
Natural gas
Natural gas is a naturally occurring gas mixture consisting primarily of methane, typically with 0–20% higher hydrocarbons . It is found associated with other hydrocarbon fuel, in coal beds, as methane clathrates, and is an important fuel source and a major feedstock for fertilizers.Most natural...
or products derived from them has been a socially significant transformation of chemical energy into other forms of energy. the energy "consumption" (one should really speak of "energy transformation") of a society or country is often quoted in reference to the average energy released by the combustion
Combustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
of these fossil fuel
Fossil fuel
Fossil fuels are fuels formed by natural processes such as anaerobic decomposition of buried dead organisms. The age of the organisms and their resulting fossil fuels is typically millions of years, and sometimes exceeds 650 million years...
s:
- 1 tonne of coal equivalent (TCE) = 29.3076 GJ = 8,141 kilowatt hour
- 1 tonne of oil equivalent (TOE) = 41.868 GJ = 11,630 kilowatt hour
On the same basis, a tank-full of gasoline
Gasoline
Gasoline , or petrol , is a toxic, translucent, petroleum-derived liquid that is primarily used as a fuel in internal combustion engines. It consists mostly of organic compounds obtained by the fractional distillation of petroleum, enhanced with a variety of additives. Some gasolines also contain...
(45 litres, 12 gallons) is equivalent to about 1.6 GJ of chemical energy. Another chemically based unit of measurement for energy is the "tonne of TNT", taken as 4.184 GJ. Hence, burning a tonne of oil releases about ten times as much energy as the explosion of one tonne of TNT: fortunately, the energy is usually released in a slower, more controlled manner.
Simple examples of storage of chemical energy are batteries and food. When food is digested and metabolized (often with oxygen), chemical energy is released, which can in turn be transformed into heat, or by muscles into kinetic energy.
According to the Bohr
Bohr
Bohr may refer to:People:* Niels Bohr , Danish atomic physicist, Nobel Prize in physics 1922* Aage Bohr , Danish nuclear physicist, Nobel Prize in physics 1975, son of Niels Bohr...
theory of the atom, the chemical energy is characterized by the Rydberg constant
Rydberg constant
The Rydberg constant, symbol R∞, named after the Swedish physicist Johannes Rydberg, is a physical constant relating to atomic spectra in the science of spectroscopy. Rydberg initially determined its value empirically from spectroscopy, but Niels Bohr later showed that its value could be calculated...
.

(see Rydberg constant
Rydberg constant
The Rydberg constant, symbol R∞, named after the Swedish physicist Johannes Rydberg, is a physical constant relating to atomic spectra in the science of spectroscopy. Rydberg initially determined its value empirically from spectroscopy, but Niels Bohr later showed that its value could be calculated...
for the meaning of the symbols).
Nuclear energy
| Nuclear binding energy is converted | |
|---|---|
| into | by |
| Mechanical energy Mechanical energy In physics, mechanical energy is the sum of potential energy and kinetic energy present in the components of a mechanical system. It is the energy associated with the motion and position of an object. The law of conservation of energy states that in an isolated system that is only subject to... |
Alpha radiation |
| Thermal energy Thermal energy Thermal energy is the part of the total internal energy of a thermodynamic system or sample of matter that results in the system's temperature.... |
Sun Sun The Sun is the star at the center of the Solar System. It is almost perfectly spherical and consists of hot plasma interwoven with magnetic fields... |
| Electrical energy | Beta radiation |
| Electromagnetic radiation Electromagnetic radiation Electromagnetic radiation is a form of energy that exhibits wave-like behavior as it travels through space... |
Gamma radiation |
| Chemical energy Chemical energy Chemical energy is the potential of a chemical substance to undergo a transformation through a chemical reaction or, to transform other chemical substances... |
Radioactive decay Radioactive decay Radioactive decay is the process by which an atomic nucleus of an unstable atom loses energy by emitting ionizing particles . The emission is spontaneous, in that the atom decays without any physical interaction with another particle from outside the atom... |
| Nuclear energy Nuclear binding energy Nuclear binding energy is the energy required to split a nucleus of an atom into its component parts. The component parts are neutrons and protons, which are collectively called nucleons... |
Nuclear isomerism |
Nuclear potential energy, along with electric potential energy
Electric potential energy
Electric potential energy, or electrostatic potential energy, is a potential energy that results from conservative Coulomb forces and is associated with the configuration of a particular set of point charges within a defined system...
, provides the energy released from nuclear fission
Nuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts , often producing free neutrons and photons , and releasing a tremendous amount of energy...
and nuclear fusion
Nuclear fusion
Nuclear fusion is the process by which two or more atomic nuclei join together, or "fuse", to form a single heavier nucleus. This is usually accompanied by the release or absorption of large quantities of energy...
processes. The result of both these processes are nuclei in which the more-optimal size of the nucleus allows the nuclear force
Nuclear force
The nuclear force is the force between two or more nucleons. It is responsible for binding of protons and neutrons into atomic nuclei. The energy released causes the masses of nuclei to be less than the total mass of the protons and neutrons which form them...
(which is opposed by the electromagnetic force) to bind nuclear particles more tightly together than before the reaction.
The Weak nuclear force (different from the strong force) provides the potential energy for certain kinds of radioactive decay, such as beta decay
Beta decay
In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a...
.
The energy released in nuclear processes is so large that the relativistic change in mass (after the energy has been removed) can be as much as several parts per thousand.
Nuclear particles (nucleon
Nucleon
In physics, a nucleon is a collective name for two particles: the neutron and the proton. These are the two constituents of the atomic nucleus. Until the 1960s, the nucleons were thought to be elementary particles...
s) like protons and neutrons are not destroyed (law of conservation of baryon number) in fission and fusion processes. A few lighter particles may be created or destroyed (example: beta minus and beta plus decay, or electron capture decay), but these minor processes are not important to the immediate energy release in fission and fusion. Rather, fission and fusion release energy when collections of baryons become more tightly bound, and it is the energy associated with a fraction of the mass of the nucleons (but not the whole particles) which appears as the heat and electromagnetic radiation generated by nuclear reactions. This heat and radiation retains the "missing" mass, but the mass is missing only because it escapes in the form of heat or light, which retain the mass and conduct it out of the system where it is not measured.
The energy from the Sun
Sun
The Sun is the star at the center of the Solar System. It is almost perfectly spherical and consists of hot plasma interwoven with magnetic fields...
, also called solar energy, is an example of this form of energy conversion. In the Sun
Sun
The Sun is the star at the center of the Solar System. It is almost perfectly spherical and consists of hot plasma interwoven with magnetic fields...
, the process of hydrogen fusion converts about 4 million metric tons of solar "matter" per second into light, which is radiated into space, but during this process, although protons change into neutrons, the number of total protons-plus-neutrons does not change. In this system, the radiated light itself (as a system) retains the "missing" mass, which represents 4 million tons per second of electromagnetic radiation, moving into space. Each of the helium nuclei which are formed in the process are less massive than the four protons from they were formed, but (to a good approximation), no particles are destroyed in the process of turning the Sun's nuclear potential energy into light. Instead, the four nucleons in a helium nucleus in the Sun have an average mass that is less than the protons which formed them, and this mass difference (4 million tons/second) is the mass that moves off as sunlight.
The nuclear binding energy
Nuclear binding energy
Nuclear binding energy is the energy required to split a nucleus of an atom into its component parts. The component parts are neutrons and protons, which are collectively called nucleons...
formula, similar to that of the chemical energy, has been found
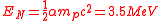
where
 is the mass of the proton
is the mass of the proton