
History of chemistry
Encyclopedia
By 1000 BC, ancient civilizations used technologies that would eventually form the basis of the various branches of chemistry
. Examples include extracting metals from ores, making pottery and glazes, fermenting beer and wine, making pigments for cosmetics and painting, extracting chemicals from plants for medicine and perfume, making cheese, dying cloth, tanning leather, rendering fat into soap, making glass, and making alloys like bronze.
Early attempts to explain the nature of matter and its transformations failed. The protoscience of chemistry, Alchemy
, was also unsuccessful in explaining the nature of matter. However, by performing experiments and recording the results the alchemist set the stage for modern chemistry. This distinction begins to emerge when a clear differentiation was made between chemistry and alchemy by Robert Boyle
in his work The Sceptical Chymist
(1661). Chemistry then becomes a full-fledged science when Antoine Lavoisier
develops his law of conservation of mass
, which demands careful measurements and quantitative observations of chemical phenomena. So, while both alchemy and chemistry are concerned with the nature of matter and its transformations, it is only the chemist
s who apply the scientific method
. The history of chemistry is intertwined with the history of thermodynamics
, especially through the work of Willard Gibbs.
. However, for millennia fire
was simply a mystical force that could transform one substance into another (burning wood, or boiling water) while producing heat and light. Fire affected many aspects of early societies. These ranged from the most simple facets of everyday life, such as cooking and habitat lighting, to more advanced technologies, such as pottery, bricks, and melting of metals to make tools.
Philosophical
attempts to rationalize why different substances have different properties (color, density, smell), exist in different states (gaseous, liquid, and solid), and react in a different manner when exposed to environments, for example to water or fire or temperature changes, led ancient philosophers to postulate the first theories on nature and chemistry. The history of such philosophical theories that relate to chemistry, can probably be traced back to every single ancient civilization. The common aspect in all these theories was the attempt to identify a small number of primary elements
that make up all the various substances in nature. Substances like air, water, and soil/earth, energy forms, such as fire and light, and more abstract concepts such as ideas, aether, and heaven, were common in ancient civilizations even in absence of any cross-fertilization; for example in Greek, Indian, Mayan, and ancient Chinese philosophies all considered air, water, earth and fire as primary elements
.
Atomism
can be traced back to ancient Greece
and ancient India
. Greek atomism dates back to 440 BC, as what might be indicated by the book De Rerum Natura (The Nature of Things) written by the Roman Lucretius
in 50 BC. In the book was found ideas traced back to Democritus
and Leucippus
, who declared that atoms were the most indivisible part of matter. This coincided with a similar declaration by Indian
philosopher Kanada
in his Vaisheshika
sutra
s around the same time period.
In much the same fashion he discussed the existence of gas
es. What Kanada declared by sutra, Democritus declared by philosophical musing. Both suffered from a lack of empirical
data. Without scientific proof, the existence of atoms was easy to deny. Aristotle
opposed the existence of atoms in 330 BC.
Much of the early development of purification methods is described by Pliny the Elder
in his Naturalis Historia
. He made attempts to explain those methods, as well as making acute observations of the state of many minerals.
and the purification of metal
s which in turn gave way to the rise of metallurgy
. During the early stages of metallurgy, methods of purification of metals were sought, and gold
, known in ancient Egypt
as early as 2600 BC, became a precious metal. The discovery of alloy
s heralded the Bronze Age
. After the Bronze Age, the history of metallurgy was marked by which army had better weaponry. Countries in Eurasia
had their heyday when they made the superior alloys, which, in turn, made better armour and better weapons. This often determined the outcomes of battles. Significant progress in metallurgy and alchemy was made in ancient India
.
 Many people were interested in finding a method that could convert cheaper metals into gold. The material that would help them do this was rumored to exist in what was called the philosopher's stone
Many people were interested in finding a method that could convert cheaper metals into gold. The material that would help them do this was rumored to exist in what was called the philosopher's stone
. This led to the protoscience
called alchemy
. Alchemy was practiced by many cultures throughout history and often contained a mixture of philosophy, mysticism, and protoscience.
Alchemy not only sought to turn base metals into gold, but especially in a Europe rocked by bubonic plague
, there was hope that alchemy would lead to the development of medicines to improve people's health. The holy grail
of this strain of alchemy was in the attempts made at finding the elixir of life
, which promised eternal youth. Neither the elixir nor the philosopher's stone were ever found. Also, characteristic of alchemists was the belief that there was in the air an "ether" which breathed life into living things. Practitioners of alchemy included Isaac Newton
, who remained one throughout his life.
Chaucer's tale exposed the more fraudulent side of alchemy, especially the manufacture of counterfeit gold from cheap substances. Less than a century earlier, Dante Alighieri
also demonstrated an awareness of this fraudulence, causing him to consign all alchemists to the Inferno in his writings. Soon after, in 1317, the Avignon
Pope John XXII
ordered all alchemists to leave France for making counterfeit money. A law was passed in England in 1403 which made the "multiplication of metals" punishable by death. Despite these and other apparently extreme measures, alchemy did not die. Royalty and privileged classes still sought to discover the philosopher's stone and the elixir of life for themselves.
There was also no agreed-upon scientific method for making experiments reproducible. Indeed many alchemists included in their methods irrelevant information such as the timing of the tides or the phases of the moon. The esoteric nature and codified vocabulary of alchemy appeared to be more useful in concealing the fact that they could not be sure of very much at all. As early as the 14th century, cracks seemed to grow in the facade of alchemy; and people became sceptical. Clearly, there needed to be a scientific method where experiments can be repeated by other people, and results needed to be reported in a clear language that laid out both what is known and unknown.
 In the Arab World
In the Arab World
, the Muslim
s were translating the works of the ancient Greeks
and Egyptians
into Arabic and were experimenting with scientific ideas. The development of the modern scientific method
was slow and arduous, but an early scientific method for chemistry began emerging among early Muslim chemists, beginning with the 9th century chemist Jābir ibn Hayyān (known as "Geber" in Europe), who is "considered as the father of chemistry". He introduced a systematic and experiment
al approach to scientific research based in the laboratory
, in contrast to the ancient Greek and Egyptian alchemists whose works were largely allegorical and often unintelligble. He also invented and named the alembic
(al-anbiq), chemically analyzed many chemical substance
s, composed lapidaries
, distinguished between alkali
s and acid
s, and manufactured hundreds of drug
s. He also refined the theory of five classical element
s into the theory of seven alchemical elements after identifying mercury
and sulfur
as chemical element
s.
Among other influential Muslim chemists, Ja'far al-Sadiq
, Alkindus
, Abū al-Rayhān al-Bīrūnī, Avicenna
and Ibn Khaldun
refuted the theories of alchemy, particularly the theory of the transmutation of metals
; and al-Tusi
described a version of the conservation of mass
, noting that a body of matter
is able to change but is not able to disappear. Rhazes refuted Aristotle
's theory of four classical element
s for the first time and set up the firm foundations of modern chemistry, using the laboratory in the modern sense, designing and describing more than twenty instruments, many parts of which are still in use today, such as a crucible, decensory, cucurbit or retort for distillation, and the head of a still with a delivery tube (ambiq, Latin alembic), and various types of furnace or stove.
 For the more honest practitioners in Europe, alchemy became an intellectual pursuit after early Arabic alchemy became available through Latin translation, and over time, they got better at it. Paracelsus
For the more honest practitioners in Europe, alchemy became an intellectual pursuit after early Arabic alchemy became available through Latin translation, and over time, they got better at it. Paracelsus
(1493–1541), for example, rejected the 4-elemental theory and with only a vague understanding of his chemicals and medicines, formed a hybrid of alchemy and science in what was to be called iatrochemistry
. Paracelsus was not perfect in making his experiments truly scientific. For example, as an extension of his theory that new compounds could be made by combining mercury with sulfur, he once made what he thought was "oil of sulfur". This was actually dimethyl ether
, which had neither mercury nor sulfur.
Practical attempts to improve the refining of ores and their extraction to smelt metals was an important source of information for early chemists, among them Georg Agricola
(1494–1555), who published his great work De re metallica
in 1556. His approach removed the mysticism associated with the subject, creating the practical base upon which others could build. The work describes the many kinds of furnace used to smelt ore, and stimulated interest in minerals and their composition. It is no coincidence that he gives numerous references to the earlier author, Pliny the Elder
and his Naturalis Historia
.
In 1605, Sir Francis Bacon published The Proficience and Advancement of Learning, which contains a description of what would later be known as the scientific method
. In 1615 Jean Beguin
publishes the Tyrocinium Chymicum
, an early chemistry textbook, and in it draws the first-ever chemical equation
.
 Robert Boyle
Robert Boyle
(1627–1691) is considered to have refined the modern scientific method for alchemy and to have separated chemistry further from alchemy. Robert Boyle was an atomist, but favoured the word corpuscle over atoms. He comments that the finest division of matter where the properties are retained is at the level of corpuscles. Boyle was credited with the discovery of Boyle's Law
. He is also credited for his landmark publication The Sceptical Chymist
, where he attempts to develop an atomic theory
of matter, with no small degree of success. He laid the foundations for the Chemical Revolution
with his mechanical
corpuscular philosophy, which in turn relied heavily on the alchemical corpuscular theory
and experimental method
dating back to the alchemist Jābir ibn Hayyān.
Despite all these advances, the person celebrated as the "father of modern chemistry" is Antoine Lavoisier
who developed his law of conservation of mass
in 1789, also called Lavoisier's Law. With this, chemistry acquired a strict quantitative nature, allowing reliable predictions to be made.
In 1754, Joseph Black
isolated carbon dioxide
, which he called "fixed air". Carl Wilhelm Scheele
and Joseph Priestley
independently isolated oxygen
, called by Priestley "dephlogisticated air" and Scheele "fire air".
Joseph Proust
proposed the law of definite proportions
, which states that elements always combine in small, whole number ratios to form compounds. In 1800, Alessandro Volta
devised the first chemical battery
, thereby founding the discipline of electrochemistry
. In 1803, John Dalton
proposed Dalton's Law
, which describes relationship between the components in a mixture of gases and the relative pressure each contributes to that of the overall mixture.
 Although the archives of chemical research draw upon work from ancient Babylonia
Although the archives of chemical research draw upon work from ancient Babylonia
, Egypt
, and especially the Arab
s and Persians
after Islam
, modern chemistry flourished from the time of Antoine Lavoisier
, who is regarded as the "father of modern chemistry", particularly for his discovery of the law of conservation of mass
, and his refutation of the phlogiston theory
of combustion
in 1783. (Phlogiston was supposed to be an imponderable substance liberated by flammable materials in burning.) Mikhail Lomonosov
independently established a tradition of chemistry in Russia
in the 18th century. Lomonosov also rejected the phlogiston theory, and anticipated the kinetic theory
of gases. He regarded heat as a form of motion, and stated the idea of conservation of matter.
) was settled, another dispute, about vitalism
and the essential distinction between organic and inorganic substances, was revolutionized by Friedrich Wöhler
's accidental synthesis of urea
from inorganic substances in 1828. Never before had an organic compound
been synthesized from inorganic material. This opened a new research field in chemistry, and by the end of the 19th century, scientists were able to synthesize hundreds of organic compounds. The most important among them are mauve
, magenta
, and other synthetic dye
s, as well as the widely used drug aspirin
. The discovery of the artificial synthesis of urea contributed greatly to the theory of isomerism, as the empirical chemical formulas for urea and ammonium
cyanate
are identical (see Wöhler synthesis
).
 Throughout the 19th century, chemistry was divided between those who followed the atomic theory of John Dalton
Throughout the 19th century, chemistry was divided between those who followed the atomic theory of John Dalton
and those who did not, such as Wilhelm Ostwald
and Ernst Mach
. Although such proponents of the atomic theory as Amedeo Avogadro
and Ludwig Boltzmann
made great advances in explaining the behavior of gas
es, this dispute was not finally settled until Jean Perrin's experimental investigation of Einstein
's atomic explanation of Brownian motion
in the first decade of the 20th century.
Well before the dispute had been settled, many had already applied the concept of atomism to chemistry. A major example was the ion
theory of Svante Arrhenius
which anticipated ideas about atomic substructure that did not fully develop until the 20th century. Michael Faraday
was another early worker, whose major contribution to chemistry was electrochemistry
, in which (among other things) a certain quantity of electricity during electrolysis
or electrodeposition
of metals was shown to be associated with certain quantities of chemical elements, and fixed quantities of the elements therefore with each other, in specific ratios. These findings, like those of Dalton's combining ratios, were early clues to the atomic nature of matter.
and Lothar Meyer's development of the periodic table
, and particularly Mendeleev's use of it to predict the existence and the properties of germanium
, gallium
, and scandium
, which Mendeleev called ekasilicon, ekaaluminium, and ekaboron
respectively. Mendeleev made his prediction in 1870; gallium was discovered in 1875, and was found to have roughly the same properties that Mendeleev predicted for it.
wrote in 1830:
However, in the second part of the 19th century, the situation changed and August Kekule wrote in 1867:
After the discovery by Ernest Rutherford
and Niels Bohr
of the atomic structure in 1912, and by Marie and Pierre Curie
of radioactivity, scientists had to change their viewpoint on the nature of matter. The experience acquired by chemists was no longer pertinent to the study of the whole nature of matter but only to aspects related to the electron cloud surrounding the atomic nuclei
and the movement of the latter in the electric field
induced by the former (see Born-Oppenheimer approximation
). The range of chemistry was thus restricted to the nature of matter around us in conditions which are not too far (or exceptionally far) from standard conditions for temperature and pressure
and in cases where the exposure to radiation is not too different from the natural microwave
, visible
or UV radiations on Earth. Chemistry was therefore re-defined as the science of matter that deals with the composition, structure, and properties of substances and with the transformations that they undergo. However the meaning of matter used here relates explicitly to substances made of atoms and molecules, disregarding the matter within the atomic nuclei and its nuclear reaction or matter within highly ionized plasmas. This does not mean that chemistry is never involved with plasma or nuclear sciences or even bosonic fields nowadays, since areas such as Quantum Chemistry and Nuclear Chemistry are currently well developed and formally recognized sub-fields of study under the Chemical sciences (Chemistry), but what is now formally recognized as subject of study under the Chemistry category as a science is always based on the use of concepts that describe or explain phenomena either from matter or to matter in the atomic or molecular scale, including the study of the behavior of many molecules as an aggregate or the study of the effects of a single proton on a single atom, but excluding phenomena that deal with different (more "exotic") types of matter (e.g. Bose-Einstein condensate, Higgs Boson, dark matter, naked singularity, etc.) and excluding principles that refer to intrinsic abstract laws of nature in which their concepts can be formulated completely without a precise formal molecular or atomic paradigmatic view (e.g. Quantum Chromodynamics, Quantum Electrodynamics, String Theory, parts of Cosmology (see Cosmochemistry
), certain areas of Nuclear Physics (see Nuclear Chemistry
),etc.). Nevertheless the field of chemistry is still, on our human scale, very broad and the claim that chemistry is everywhere is accurate.
and its application to the hydrogen atom
in 1926. However, the 1927 article of Walter Heitler
and Fritz London
is often recognised as the first milestone in the history of quantum chemistry. This is the first application of quantum mechanics
to the diatomic hydrogen
molecule, and thus to the phenomenon of the chemical bond
. In the following years much progress was accomplished by Edward Teller
, Robert S. Mulliken
, Max Born
, J. Robert Oppenheimer, Linus Pauling
, Erich Hückel
, Douglas Hartree
, Vladimir Aleksandrovich Fock, to cite a few.
Still, skepticism remained as to the general power of quantum mechanics applied to complex chemical systems. The situation around 1930 is described by Paul Dirac
:
Hence the quantum mechanical methods developed in the 1930s and 1940s are often referred to as theoretical molecular
or atomic physics
to underline the fact that they were more the application of quantum mechanics to chemistry and spectroscopy
than answers to chemically relevant questions.
In the 1940s many physicists turned from molecular
or atomic physics
to nuclear physics
(like J. Robert Oppenheimer or Edward Teller
). In 1951, a milestone article in quantum chemistry is the seminal paper of Clemens C. J. Roothaan
on Roothaan equations
. It opened the avenue to the solution of the self-consistent field equations for small molecules like hydrogen
or nitrogen
. Those computations were performed with the help of tables of integrals which were computed on the most advanced computers of the time.
ic structure of the atom
; Linus Pauling
's book on The Nature of the Chemical Bond used the principles of quantum mechanics to deduce bond angles in ever-more complicated molecules. However, though some principles deduced from quantum mechanics were able to predict qualitatively some chemical features for biologically relevant molecules, they were, till the end of the 20th century, more a collection of rules, observations, and recipes than rigorous ab initio
quantitative methods.
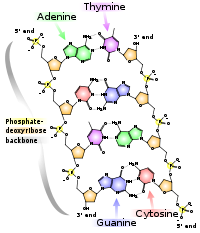 This heuristic approach triumphed in 1953 when James Watson
This heuristic approach triumphed in 1953 when James Watson
and Francis Crick
deduced the double helical structure of DNA
by constructing models constrained by and informed by the knowledge of the chemistry of the constituent parts and the X-ray diffraction patterns obtained by Rosalind Franklin
. This discovery lead to an explosion of research into the biochemistry
of life.
In the same year, the Miller-Urey experiment
demonstrated that basic constituents of protein
, simple amino acid
s, could themselves be built up from simpler molecules in a simulation
of primordial processes
on Earth. Though many questions remain about the true nature of the origin of life, this was the first attempt by chemists to study hypothetical processes in the laboratory under controlled conditions.
In 1983 Kary Mullis
devised a method for the in-vitro amplification of DNA, known as the polymerase chain reaction
(PCR), which revolutionized the chemical processes used in the laboratory to manipulate it. PCR could be used to synthesize specific pieces of DNA and made possible the sequencing of DNA
of organisms, which culminated in the huge human genome project
.
An important piece in the double helix puzzle was solved by one of Pauling's student Matthew Meselson
and Frank Stahl, the result of their collaboration (Meselson-Stahl experiment
) has been called as "the most beautiful experiment in biology".
They used a centrifugation technique that sorted molecules according to differences in weight. Because nitrogen atoms are a component of DNA, they were labelled and therefore tracked in replication in bacteria.
extracted from the earth for the production of a host of chemicals and largely replaced the use of whale oil
, coal tar
and naval stores
used previously. Large scale production and refinement of petroleum
provided feedstocks for liquid fuels
such as gasoline
and diesel, solvents, lubricants, asphalt
, waxes, and for the production of many of the common materials of the modern world, such as synthetic fibers, plastic
s, paints, detergent
s, pharmaceuticals, adhesives and ammonia
as fertilizer
and for other uses. Many of these required new catalysts and the utilization of chemical engineering
for their cost-effective production.
In the mid-twentieth century, control of the electronic structure of semiconductor
materials was made precise by the creation of large ingots of extremely pure single crystals of silicon
and germanium
. Accurate control of their chemical composition by doping with other elements made the production of the solid state transistor
in 1951 and made possible the production of tiny integrated circuit
s for use in electronic devices, especially computers.
Documentaries
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
. Examples include extracting metals from ores, making pottery and glazes, fermenting beer and wine, making pigments for cosmetics and painting, extracting chemicals from plants for medicine and perfume, making cheese, dying cloth, tanning leather, rendering fat into soap, making glass, and making alloys like bronze.
Early attempts to explain the nature of matter and its transformations failed. The protoscience of chemistry, Alchemy
Alchemy
Alchemy is an influential philosophical tradition whose early practitioners’ claims to profound powers were known from antiquity. The defining objectives of alchemy are varied; these include the creation of the fabled philosopher's stone possessing powers including the capability of turning base...
, was also unsuccessful in explaining the nature of matter. However, by performing experiments and recording the results the alchemist set the stage for modern chemistry. This distinction begins to emerge when a clear differentiation was made between chemistry and alchemy by Robert Boyle
Robert Boyle
Robert Boyle FRS was a 17th century natural philosopher, chemist, physicist, and inventor, also noted for his writings in theology. He has been variously described as English, Irish, or Anglo-Irish, his father having come to Ireland from England during the time of the English plantations of...
in his work The Sceptical Chymist
The Sceptical Chymist
The Sceptical Chymist: or Chymico-Physical Doubts & Paradoxes is the title of Robert Boyle's masterpiece of scientific literature, published in London in 1661. In the form of a dialogue, the Sceptical Chymist presented Boyle's hypothesis that matter consisted of atoms and clusters of atoms in...
(1661). Chemistry then becomes a full-fledged science when Antoine Lavoisier
Antoine Lavoisier
Antoine-Laurent de Lavoisier , the "father of modern chemistry", was a French nobleman prominent in the histories of chemistry and biology...
develops his law of conservation of mass
Conservation of mass
The law of conservation of mass, also known as the principle of mass/matter conservation, states that the mass of an isolated system will remain constant over time...
, which demands careful measurements and quantitative observations of chemical phenomena. So, while both alchemy and chemistry are concerned with the nature of matter and its transformations, it is only the chemist
Chemist
A chemist is a scientist trained in the study of chemistry. Chemists study the composition of matter and its properties such as density and acidity. Chemists carefully describe the properties they study in terms of quantities, with detail on the level of molecules and their component atoms...
s who apply the scientific method
Scientific method
Scientific method refers to a body of techniques for investigating phenomena, acquiring new knowledge, or correcting and integrating previous knowledge. To be termed scientific, a method of inquiry must be based on gathering empirical and measurable evidence subject to specific principles of...
. The history of chemistry is intertwined with the history of thermodynamics
History of thermodynamics
The history of thermodynamics is a fundamental strand in the history of physics, the history of chemistry, and the history of science in general...
, especially through the work of Willard Gibbs.
From fire to atomism
Arguably the first chemical reaction used in a controlled manner was fireCombustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
. However, for millennia fire
Fire
Fire is the rapid oxidation of a material in the chemical process of combustion, releasing heat, light, and various reaction products. Slower oxidative processes like rusting or digestion are not included by this definition....
was simply a mystical force that could transform one substance into another (burning wood, or boiling water) while producing heat and light. Fire affected many aspects of early societies. These ranged from the most simple facets of everyday life, such as cooking and habitat lighting, to more advanced technologies, such as pottery, bricks, and melting of metals to make tools.
Philosophical
Philosophy
Philosophy is the study of general and fundamental problems, such as those connected with existence, knowledge, values, reason, mind, and language. Philosophy is distinguished from other ways of addressing such problems by its critical, generally systematic approach and its reliance on rational...
attempts to rationalize why different substances have different properties (color, density, smell), exist in different states (gaseous, liquid, and solid), and react in a different manner when exposed to environments, for example to water or fire or temperature changes, led ancient philosophers to postulate the first theories on nature and chemistry. The history of such philosophical theories that relate to chemistry, can probably be traced back to every single ancient civilization. The common aspect in all these theories was the attempt to identify a small number of primary elements
Classical element
Many philosophies and worldviews have a set of classical elements believed to reflect the simplest essential parts and principles of which anything consists or upon which the constitution and fundamental powers of anything are based. Most frequently, classical elements refer to ancient beliefs...
that make up all the various substances in nature. Substances like air, water, and soil/earth, energy forms, such as fire and light, and more abstract concepts such as ideas, aether, and heaven, were common in ancient civilizations even in absence of any cross-fertilization; for example in Greek, Indian, Mayan, and ancient Chinese philosophies all considered air, water, earth and fire as primary elements
Classical element
Many philosophies and worldviews have a set of classical elements believed to reflect the simplest essential parts and principles of which anything consists or upon which the constitution and fundamental powers of anything are based. Most frequently, classical elements refer to ancient beliefs...
.
Atomism
Atomism
Atomism is a natural philosophy that developed in several ancient traditions. The atomists theorized that the natural world consists of two fundamental parts: indivisible atoms and empty void.According to Aristotle, atoms are indestructible and immutable and there are an infinite variety of shapes...
can be traced back to ancient Greece
Ancient Greece
Ancient Greece is a civilization belonging to a period of Greek history that lasted from the Archaic period of the 8th to 6th centuries BC to the end of antiquity. Immediately following this period was the beginning of the Early Middle Ages and the Byzantine era. Included in Ancient Greece is the...
and ancient India
History of India
The history of India begins with evidence of human activity of Homo sapiens as long as 75,000 years ago, or with earlier hominids including Homo erectus from about 500,000 years ago. The Indus Valley Civilization, which spread and flourished in the northwestern part of the Indian subcontinent from...
. Greek atomism dates back to 440 BC, as what might be indicated by the book De Rerum Natura (The Nature of Things) written by the Roman Lucretius
Lucretius
Titus Lucretius Carus was a Roman poet and philosopher. His only known work is an epic philosophical poem laying out the beliefs of Epicureanism, De rerum natura, translated into English as On the Nature of Things or "On the Nature of the Universe".Virtually no details have come down concerning...
in 50 BC. In the book was found ideas traced back to Democritus
Democritus
Democritus was an Ancient Greek philosopher born in Abdera, Thrace, Greece. He was an influential pre-Socratic philosopher and pupil of Leucippus, who formulated an atomic theory for the cosmos....
and Leucippus
Leucippus
Leucippus or Leukippos was one of the earliest Greeks to develop the theory of atomism — the idea that everything is composed entirely of various imperishable, indivisible elements called atoms — which was elaborated in greater detail by his pupil and successor, Democritus...
, who declared that atoms were the most indivisible part of matter. This coincided with a similar declaration by Indian
Indian philosophy
India has a rich and diverse philosophical tradition dating back to ancient times. According to Radhakrishnan, the earlier Upanisads constitute "...the earliest philosophical compositions of the world."...
philosopher Kanada
Kanada
It has been claimed that Kashyapa, later known as Kanada was a Hindu sage and philosopher who founded the philosophical school of Vaisheshika. He talked of Dvyanuka and tryanuka...
in his Vaisheshika
Vaisheshika
Vaisheshika or ' is one of the six Hindu schools of philosophy of India. Historically, it has been closely associated with the Hindu school of logic, Nyaya....
sutra
Sutra
Sūtra is an aphorism or a collection of such aphorisms in the form of a manual. Literally it means a thread or line that holds things together and is derived from the verbal root siv-, meaning to sew , as does the medical term...
s around the same time period.
In much the same fashion he discussed the existence of gas
Gas
Gas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
es. What Kanada declared by sutra, Democritus declared by philosophical musing. Both suffered from a lack of empirical
Empiricism
Empiricism is a theory of knowledge that asserts that knowledge comes only or primarily via sensory experience. One of several views of epistemology, the study of human knowledge, along with rationalism, idealism and historicism, empiricism emphasizes the role of experience and evidence,...
data. Without scientific proof, the existence of atoms was easy to deny. Aristotle
Aristotle
Aristotle was a Greek philosopher and polymath, a student of Plato and teacher of Alexander the Great. His writings cover many subjects, including physics, metaphysics, poetry, theater, music, logic, rhetoric, linguistics, politics, government, ethics, biology, and zoology...
opposed the existence of atoms in 330 BC.
Much of the early development of purification methods is described by Pliny the Elder
Pliny the Elder
Gaius Plinius Secundus , better known as Pliny the Elder, was a Roman author, naturalist, and natural philosopher, as well as naval and army commander of the early Roman Empire, and personal friend of the emperor Vespasian...
in his Naturalis Historia
Naturalis Historia
The Natural History is an encyclopedia published circa AD 77–79 by Pliny the Elder. It is one of the largest single works to have survived from the Roman Empire to the modern day and purports to cover the entire field of ancient knowledge, based on the best authorities available to Pliny...
. He made attempts to explain those methods, as well as making acute observations of the state of many minerals.
The rise of metallurgy
It was fire that led to the discovery of glassGlass
Glass is an amorphous solid material. Glasses are typically brittle and optically transparent.The most familiar type of glass, used for centuries in windows and drinking vessels, is soda-lime glass, composed of about 75% silica plus Na2O, CaO, and several minor additives...
and the purification of metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
s which in turn gave way to the rise of metallurgy
Metallurgy
Metallurgy is a domain of materials science that studies the physical and chemical behavior of metallic elements, their intermetallic compounds, and their mixtures, which are called alloys. It is also the technology of metals: the way in which science is applied to their practical use...
. During the early stages of metallurgy, methods of purification of metals were sought, and gold
Gold
Gold is a chemical element with the symbol Au and an atomic number of 79. Gold is a dense, soft, shiny, malleable and ductile metal. Pure gold has a bright yellow color and luster traditionally considered attractive, which it maintains without oxidizing in air or water. Chemically, gold is a...
, known in ancient Egypt
Ancient Egypt
Ancient Egypt was an ancient civilization of Northeastern Africa, concentrated along the lower reaches of the Nile River in what is now the modern country of Egypt. Egyptian civilization coalesced around 3150 BC with the political unification of Upper and Lower Egypt under the first pharaoh...
as early as 2600 BC, became a precious metal. The discovery of alloy
Alloy
An alloy is a mixture or metallic solid solution composed of two or more elements. Complete solid solution alloys give single solid phase microstructure, while partial solutions give two or more phases that may or may not be homogeneous in distribution, depending on thermal history...
s heralded the Bronze Age
Bronze Age
The Bronze Age is a period characterized by the use of copper and its alloy bronze as the chief hard materials in the manufacture of some implements and weapons. Chronologically, it stands between the Stone Age and Iron Age...
. After the Bronze Age, the history of metallurgy was marked by which army had better weaponry. Countries in Eurasia
Eurasia
Eurasia is a continent or supercontinent comprising the traditional continents of Europe and Asia ; covering about 52,990,000 km2 or about 10.6% of the Earth's surface located primarily in the eastern and northern hemispheres...
had their heyday when they made the superior alloys, which, in turn, made better armour and better weapons. This often determined the outcomes of battles. Significant progress in metallurgy and alchemy was made in ancient India
History of India
The history of India begins with evidence of human activity of Homo sapiens as long as 75,000 years ago, or with earlier hominids including Homo erectus from about 500,000 years ago. The Indus Valley Civilization, which spread and flourished in the northwestern part of the Indian subcontinent from...
.
The philosopher's stone and the rise of alchemy

Philosopher's stone
The philosopher's stone is a legendary alchemical substance said to be capable of turning base metals into gold or silver. It was also sometimes believed to be an elixir of life, useful for rejuvenation and possibly for achieving immortality. For many centuries, it was the most sought-after goal...
. This led to the protoscience
Protoscience
In the philosophy of science, a protoscience is an area of scientific endeavor that is in the process of becoming established. Protoscience is distinguished from pseudoscience by its standard practices of good science, such as a willingness to be disproven by new evidence, or to be replaced by a...
called alchemy
Alchemy
Alchemy is an influential philosophical tradition whose early practitioners’ claims to profound powers were known from antiquity. The defining objectives of alchemy are varied; these include the creation of the fabled philosopher's stone possessing powers including the capability of turning base...
. Alchemy was practiced by many cultures throughout history and often contained a mixture of philosophy, mysticism, and protoscience.
Alchemy not only sought to turn base metals into gold, but especially in a Europe rocked by bubonic plague
Bubonic plague
Plague is a deadly infectious disease that is caused by the enterobacteria Yersinia pestis, named after the French-Swiss bacteriologist Alexandre Yersin. Primarily carried by rodents and spread to humans via fleas, the disease is notorious throughout history, due to the unrivaled scale of death...
, there was hope that alchemy would lead to the development of medicines to improve people's health. The holy grail
Holy Grail
The Holy Grail is a sacred object figuring in literature and certain Christian traditions, most often identified with the dish, plate, or cup used by Jesus at the Last Supper and said to possess miraculous powers...
of this strain of alchemy was in the attempts made at finding the elixir of life
Elixir of life
The elixir of life, also known as the elixir of immortality and sometimes equated with the philosopher's stone, is a legendary potion, or drink, that grants the drinker eternal life and or eternal youth. Many practitioners of alchemy pursued it. The elixir of life was also said to be able to create...
, which promised eternal youth. Neither the elixir nor the philosopher's stone were ever found. Also, characteristic of alchemists was the belief that there was in the air an "ether" which breathed life into living things. Practitioners of alchemy included Isaac Newton
Isaac Newton
Sir Isaac Newton PRS was an English physicist, mathematician, astronomer, natural philosopher, alchemist, and theologian, who has been "considered by many to be the greatest and most influential scientist who ever lived."...
, who remained one throughout his life.
Problems encountered with alchemy
There were several problems with alchemy, as seen from today's standpoint. There was no systematic naming system for new compounds, and the language was esoteric and vague to the point that the terminologies meant different things to different people. In fact, according to The Fontana History of Chemistry (Brock, 1992):
The language of alchemy soon developed an arcane and secretive technical vocabulary designed to conceal information from the uninitiated. To a large degree, this language is incomprehensible to us today, though it is apparent that readers of Geoffery Chaucer's Canon's Yeoman's TaleThe Canon's Yeoman's Prologue and TaleThe Canon's Yeoman's Tale is one of The Canterbury Tales by Geoffrey Chaucer.The Canon and his Yeoman are not mentioned in the General Prologue of The Canterbury Tales, where most of the other pilgrims are described, but they arrive later after riding fast to catch up with the group. The tale the...
or audiences of Ben JonsonBen JonsonBenjamin Jonson was an English Renaissance dramatist, poet and actor. A contemporary of William Shakespeare, he is best known for his satirical plays, particularly Volpone, The Alchemist, and Bartholomew Fair, which are considered his best, and his lyric poems...
's The AlchemistThe Alchemist (play)The Alchemist is a comedy by English playwright Ben Jonson. First performed in 1610 by the King's Men, it is generally considered Jonson's best and most characteristic comedy; Samuel Taylor Coleridge claimed that it had one of the three most perfect plots in literature...
were able to construe it sufficiently to laugh at it.
Chaucer's tale exposed the more fraudulent side of alchemy, especially the manufacture of counterfeit gold from cheap substances. Less than a century earlier, Dante Alighieri
Dante Alighieri
Durante degli Alighieri, mononymously referred to as Dante , was an Italian poet, prose writer, literary theorist, moral philosopher, and political thinker. He is best known for the monumental epic poem La commedia, later named La divina commedia ...
also demonstrated an awareness of this fraudulence, causing him to consign all alchemists to the Inferno in his writings. Soon after, in 1317, the Avignon
Avignon
Avignon is a French commune in southeastern France in the départment of the Vaucluse bordered by the left bank of the Rhône river. Of the 94,787 inhabitants of the city on 1 January 2010, 12 000 live in the ancient town centre surrounded by its medieval ramparts.Often referred to as the...
Pope John XXII
Pope John XXII
Pope John XXII , born Jacques Duèze , was pope from 1316 to 1334. He was the second Pope of the Avignon Papacy , elected by a conclave in Lyon assembled by Philip V of France...
ordered all alchemists to leave France for making counterfeit money. A law was passed in England in 1403 which made the "multiplication of metals" punishable by death. Despite these and other apparently extreme measures, alchemy did not die. Royalty and privileged classes still sought to discover the philosopher's stone and the elixir of life for themselves.
There was also no agreed-upon scientific method for making experiments reproducible. Indeed many alchemists included in their methods irrelevant information such as the timing of the tides or the phases of the moon. The esoteric nature and codified vocabulary of alchemy appeared to be more useful in concealing the fact that they could not be sure of very much at all. As early as the 14th century, cracks seemed to grow in the facade of alchemy; and people became sceptical. Clearly, there needed to be a scientific method where experiments can be repeated by other people, and results needed to be reported in a clear language that laid out both what is known and unknown.
From alchemy to chemistry
Early chemists

Arab world
The Arab world refers to Arabic-speaking states, territories and populations in North Africa, Western Asia and elsewhere.The standard definition of the Arab world comprises the 22 states and territories of the Arab League stretching from the Atlantic Ocean in the west to the Arabian Sea in the...
, the Muslim
Muslim
A Muslim, also spelled Moslem, is an adherent of Islam, a monotheistic, Abrahamic religion based on the Quran, which Muslims consider the verbatim word of God as revealed to prophet Muhammad. "Muslim" is the Arabic term for "submitter" .Muslims believe that God is one and incomparable...
s were translating the works of the ancient Greeks
Ancient Greece
Ancient Greece is a civilization belonging to a period of Greek history that lasted from the Archaic period of the 8th to 6th centuries BC to the end of antiquity. Immediately following this period was the beginning of the Early Middle Ages and the Byzantine era. Included in Ancient Greece is the...
and Egyptians
Ancient Egypt
Ancient Egypt was an ancient civilization of Northeastern Africa, concentrated along the lower reaches of the Nile River in what is now the modern country of Egypt. Egyptian civilization coalesced around 3150 BC with the political unification of Upper and Lower Egypt under the first pharaoh...
into Arabic and were experimenting with scientific ideas. The development of the modern scientific method
Scientific method
Scientific method refers to a body of techniques for investigating phenomena, acquiring new knowledge, or correcting and integrating previous knowledge. To be termed scientific, a method of inquiry must be based on gathering empirical and measurable evidence subject to specific principles of...
was slow and arduous, but an early scientific method for chemistry began emerging among early Muslim chemists, beginning with the 9th century chemist Jābir ibn Hayyān (known as "Geber" in Europe), who is "considered as the father of chemistry". He introduced a systematic and experiment
Experiment
An experiment is a methodical procedure carried out with the goal of verifying, falsifying, or establishing the validity of a hypothesis. Experiments vary greatly in their goal and scale, but always rely on repeatable procedure and logical analysis of the results...
al approach to scientific research based in the laboratory
Laboratory
A laboratory is a facility that provides controlled conditions in which scientific research, experiments, and measurement may be performed. The title of laboratory is also used for certain other facilities where the processes or equipment used are similar to those in scientific laboratories...
, in contrast to the ancient Greek and Egyptian alchemists whose works were largely allegorical and often unintelligble. He also invented and named the alembic
Alembic
An alembic is an alchemical still consisting of two vessels connected by a tube...
(al-anbiq), chemically analyzed many chemical substance
Chemical substance
In chemistry, a chemical substance is a form of matter that has constant chemical composition and characteristic properties. It cannot be separated into components by physical separation methods, i.e. without breaking chemical bonds. They can be solids, liquids or gases.Chemical substances are...
s, composed lapidaries
Lapidary
A lapidary is an artist or artisan who forms stone, mineral, gemstones, and other suitably durable materials into decorative items such as engraved gems, including cameos, or cabochons, and faceted designs...
, distinguished between alkali
Alkali
In chemistry, an alkali is a basic, ionic salt of an alkali metal or alkaline earth metal element. Some authors also define an alkali as a base that dissolves in water. A solution of a soluble base has a pH greater than 7. The adjective alkaline is commonly used in English as a synonym for base,...
s and acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
s, and manufactured hundreds of drug
Drug
A drug, broadly speaking, is any substance that, when absorbed into the body of a living organism, alters normal bodily function. There is no single, precise definition, as there are different meanings in drug control law, government regulations, medicine, and colloquial usage.In pharmacology, a...
s. He also refined the theory of five classical element
Classical element
Many philosophies and worldviews have a set of classical elements believed to reflect the simplest essential parts and principles of which anything consists or upon which the constitution and fundamental powers of anything are based. Most frequently, classical elements refer to ancient beliefs...
s into the theory of seven alchemical elements after identifying mercury
Mercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum...
and sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
as chemical element
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
s.
Among other influential Muslim chemists, Ja'far al-Sadiq
Ja'far al-Sadiq
Jaʿfar ibn Muhammad al-Sādiq was a descendant of Muhammad and a prominent Muslim jurist. He is revered as an Imam by the adherents of Shi'a Islam and as a renowned Islamic scholar and personality by Sunni Muslims. The Shi'a Muslims consider him to be the sixth Imam or leader and spiritual...
, Alkindus
Al-Kindi
' , known as "the Philosopher of the Arabs", was a Muslim Arab philosopher, mathematician, physician, and musician. Al-Kindi was the first of the Muslim peripatetic philosophers, and is unanimously hailed as the "father of Islamic or Arabic philosophy" for his synthesis, adaptation and promotion...
, Abū al-Rayhān al-Bīrūnī, Avicenna
Avicenna
Abū ʿAlī al-Ḥusayn ibn ʿAbd Allāh ibn Sīnā , commonly known as Ibn Sīnā or by his Latinized name Avicenna, was a Persian polymath, who wrote almost 450 treatises on a wide range of subjects, of which around 240 have survived...
and Ibn Khaldun
Ibn Khaldun
Ibn Khaldūn or Ibn Khaldoun was an Arab Tunisian historiographer and historian who is often viewed as one of the forerunners of modern historiography, sociology and economics...
refuted the theories of alchemy, particularly the theory of the transmutation of metals
Philosopher's stone
The philosopher's stone is a legendary alchemical substance said to be capable of turning base metals into gold or silver. It was also sometimes believed to be an elixir of life, useful for rejuvenation and possibly for achieving immortality. For many centuries, it was the most sought-after goal...
; and al-Tusi
Nasir al-Din al-Tusi
Khawaja Muḥammad ibn Muḥammad ibn Ḥasan Ṭūsī , better known as Naṣīr al-Dīn al-Ṭūsī , was a Persian polymath and prolific writer: an astronomer, biologist, chemist, mathematician, philosopher, physician, physicist, scientist, theologian and Marja Taqleed...
described a version of the conservation of mass
Conservation of mass
The law of conservation of mass, also known as the principle of mass/matter conservation, states that the mass of an isolated system will remain constant over time...
, noting that a body of matter
Matter
Matter is a general term for the substance of which all physical objects consist. Typically, matter includes atoms and other particles which have mass. A common way of defining matter is as anything that has mass and occupies volume...
is able to change but is not able to disappear. Rhazes refuted Aristotle
Aristotle
Aristotle was a Greek philosopher and polymath, a student of Plato and teacher of Alexander the Great. His writings cover many subjects, including physics, metaphysics, poetry, theater, music, logic, rhetoric, linguistics, politics, government, ethics, biology, and zoology...
's theory of four classical element
Classical element
Many philosophies and worldviews have a set of classical elements believed to reflect the simplest essential parts and principles of which anything consists or upon which the constitution and fundamental powers of anything are based. Most frequently, classical elements refer to ancient beliefs...
s for the first time and set up the firm foundations of modern chemistry, using the laboratory in the modern sense, designing and describing more than twenty instruments, many parts of which are still in use today, such as a crucible, decensory, cucurbit or retort for distillation, and the head of a still with a delivery tube (ambiq, Latin alembic), and various types of furnace or stove.

Paracelsus
Paracelsus was a German-Swiss Renaissance physician, botanist, alchemist, astrologer, and general occultist....
(1493–1541), for example, rejected the 4-elemental theory and with only a vague understanding of his chemicals and medicines, formed a hybrid of alchemy and science in what was to be called iatrochemistry
Iatrochemistry
Iatrochemistry is a branch of both chemistry and medicine. Having its roots in alchemy, iatrochemistry seeks to provide chemical solutions to diseases and medical ailments....
. Paracelsus was not perfect in making his experiments truly scientific. For example, as an extension of his theory that new compounds could be made by combining mercury with sulfur, he once made what he thought was "oil of sulfur". This was actually dimethyl ether
Dimethyl ether
Dimethyl ether , also known as methoxymethane, is the organic compound with the formula . The simplest ether, it is a colourless gas that is a useful precursor to other organic compounds and an aerosol propellant. When combusted, DME produces minimal soot and CO, though HC and NOx formation is...
, which had neither mercury nor sulfur.
Practical attempts to improve the refining of ores and their extraction to smelt metals was an important source of information for early chemists, among them Georg Agricola
Georg Agricola
Georgius Agricola was a German scholar and scientist. Known as "the father of mineralogy", he was born at Glauchau in Saxony. His real name was Georg Pawer; Agricola is the Latinised version of his name, Pawer meaning "farmer"...
(1494–1555), who published his great work De re metallica
De re metallica
De re metallica is a book cataloguing the state of the art of mining, refining, and smelting metals, published in 1556. The author was Georg Bauer, whose pen name was the Latinized Georgius Agricola...
in 1556. His approach removed the mysticism associated with the subject, creating the practical base upon which others could build. The work describes the many kinds of furnace used to smelt ore, and stimulated interest in minerals and their composition. It is no coincidence that he gives numerous references to the earlier author, Pliny the Elder
Pliny the Elder
Gaius Plinius Secundus , better known as Pliny the Elder, was a Roman author, naturalist, and natural philosopher, as well as naval and army commander of the early Roman Empire, and personal friend of the emperor Vespasian...
and his Naturalis Historia
Naturalis Historia
The Natural History is an encyclopedia published circa AD 77–79 by Pliny the Elder. It is one of the largest single works to have survived from the Roman Empire to the modern day and purports to cover the entire field of ancient knowledge, based on the best authorities available to Pliny...
.
In 1605, Sir Francis Bacon published The Proficience and Advancement of Learning, which contains a description of what would later be known as the scientific method
Scientific method
Scientific method refers to a body of techniques for investigating phenomena, acquiring new knowledge, or correcting and integrating previous knowledge. To be termed scientific, a method of inquiry must be based on gathering empirical and measurable evidence subject to specific principles of...
. In 1615 Jean Beguin
Jean Beguin
Jean Beguin was an iatrochemist noted for his 1610 Tyrocinium Chymicum , which many consider to be one of the first chemistry textbooks. In the 1615 edition of his textbook, Beguin made the first-ever chemical equation or rudimentary reaction diagrams, showing the results of reactions in which...
publishes the Tyrocinium Chymicum
Tyrocinium Chymicum
Tyrocinium Chymicum was a published set of chemistry lecture notes started by Jean Beguin in 1610 in Paris, France. It has been suggested that it was the first chemistry text book...
, an early chemistry textbook, and in it draws the first-ever chemical equation
Chemical equation
A chemical equation is the symbolic representation of a chemical reaction where the reactant entities are given on the left hand side and the product entities on the right hand side. The coefficients next to the symbols and formulae of entities are the absolute values of the stoichiometric numbers...
.

Robert Boyle
Robert Boyle FRS was a 17th century natural philosopher, chemist, physicist, and inventor, also noted for his writings in theology. He has been variously described as English, Irish, or Anglo-Irish, his father having come to Ireland from England during the time of the English plantations of...
(1627–1691) is considered to have refined the modern scientific method for alchemy and to have separated chemistry further from alchemy. Robert Boyle was an atomist, but favoured the word corpuscle over atoms. He comments that the finest division of matter where the properties are retained is at the level of corpuscles. Boyle was credited with the discovery of Boyle's Law
Boyle's law
Boyle's law is one of many gas laws and a special case of the ideal gas law. Boyle's law describes the inversely proportional relationship between the absolute pressure and volume of a gas, if the temperature is kept constant within a closed system...
. He is also credited for his landmark publication The Sceptical Chymist
The Sceptical Chymist
The Sceptical Chymist: or Chymico-Physical Doubts & Paradoxes is the title of Robert Boyle's masterpiece of scientific literature, published in London in 1661. In the form of a dialogue, the Sceptical Chymist presented Boyle's hypothesis that matter consisted of atoms and clusters of atoms in...
, where he attempts to develop an atomic theory
Atomic theory
In chemistry and physics, atomic theory is a theory of the nature of matter, which states that matter is composed of discrete units called atoms, as opposed to the obsolete notion that matter could be divided into any arbitrarily small quantity...
of matter, with no small degree of success. He laid the foundations for the Chemical Revolution
Chemical Revolution
The chemical revolution, also called the first chemical revolution, denotes the reformulation of chemistry based on the Law of Conservation of Matter and the oxygen theory of combustion. It was centered on the work of French chemist Antoine Lavoisier...
with his mechanical
Mechanism (philosophy)
Mechanism is the belief that natural wholes are like machines or artifacts, composed of parts lacking any intrinsic relationship to each other, and with their order imposed from without. Thus, the source of an apparent thing's activities is not the whole itself, but its parts or an external...
corpuscular philosophy, which in turn relied heavily on the alchemical corpuscular theory
Corpuscularianism
Corpuscularianism is a physical theory that supposed all matter to be composed of minute particles, which became important in the Seventeenth century. Among the leading corpuscularians were Rene Descartes, Robert Boyle, and John Locke....
and experimental method
Scientific method
Scientific method refers to a body of techniques for investigating phenomena, acquiring new knowledge, or correcting and integrating previous knowledge. To be termed scientific, a method of inquiry must be based on gathering empirical and measurable evidence subject to specific principles of...
dating back to the alchemist Jābir ibn Hayyān.
Despite all these advances, the person celebrated as the "father of modern chemistry" is Antoine Lavoisier
Antoine Lavoisier
Antoine-Laurent de Lavoisier , the "father of modern chemistry", was a French nobleman prominent in the histories of chemistry and biology...
who developed his law of conservation of mass
Conservation of mass
The law of conservation of mass, also known as the principle of mass/matter conservation, states that the mass of an isolated system will remain constant over time...
in 1789, also called Lavoisier's Law. With this, chemistry acquired a strict quantitative nature, allowing reliable predictions to be made.
In 1754, Joseph Black
Joseph Black
Joseph Black FRSE FRCPE FPSG was a Scottish physician and chemist, known for his discoveries of latent heat, specific heat, and carbon dioxide. He was professor of Medicine at University of Glasgow . James Watt, who was appointed as philosophical instrument maker at the same university...
isolated carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
, which he called "fixed air". Carl Wilhelm Scheele
Carl Wilhelm Scheele
Carl Wilhelm Scheele was a German-Swedish pharmaceutical chemist. Isaac Asimov called him "hard-luck Scheele" because he made a number of chemical discoveries before others who are generally given the credit...
and Joseph Priestley
Joseph Priestley
Joseph Priestley, FRS was an 18th-century English theologian, Dissenting clergyman, natural philosopher, chemist, educator, and political theorist who published over 150 works...
independently isolated oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
, called by Priestley "dephlogisticated air" and Scheele "fire air".
Joseph Proust
Joseph Proust
Joseph Louis Proust was a French chemist.-Life:Joseph L. Proust was born on September 26, 1754 in Angers, France. His father served as an apothecary in Angers. Joseph studied chemistry in his father’s shop and later came to Paris where he gained the appointment of apothecary in chief to the...
proposed the law of definite proportions
Law of definite proportions
In chemistry, the law of definite proportions, sometimes called Proust's Law, states that a chemical compound always contains exactly the same proportion of elements by mass. An equivalent statement is the law of constant composition, which states that all samples of a given chemical compound have...
, which states that elements always combine in small, whole number ratios to form compounds. In 1800, Alessandro Volta
Alessandro Volta
Count Alessandro Giuseppe Antonio Anastasio Gerolamo Umberto Volta was a Lombard physicist known especially for the invention of the battery in 1800.-Early life and works:...
devised the first chemical battery
Voltaic pile
A voltaic pile is a set of individual Galvanic cells placed in series. The voltaic pile, invented by Alessandro Volta in 1800, was the first electric battery...
, thereby founding the discipline of electrochemistry
Electrochemistry
Electrochemistry is a branch of chemistry that studies chemical reactions which take place in a solution at the interface of an electron conductor and an ionic conductor , and which involve electron transfer between the electrode and the electrolyte or species in solution.If a chemical reaction is...
. In 1803, John Dalton
John Dalton
John Dalton FRS was an English chemist, meteorologist and physicist. He is best known for his pioneering work in the development of modern atomic theory, and his research into colour blindness .-Early life:John Dalton was born into a Quaker family at Eaglesfield, near Cockermouth, Cumberland,...
proposed Dalton's Law
Dalton's law
In chemistry and physics, Dalton's law states that the total pressure exerted by a gaseous mixture is equal to the sum of the partial pressures of each individual component in a gas mixture...
, which describes relationship between the components in a mixture of gases and the relative pressure each contributes to that of the overall mixture.
Antoine Lavoisier

Babylonia
Babylonia was an ancient cultural region in central-southern Mesopotamia , with Babylon as its capital. Babylonia emerged as a major power when Hammurabi Babylonia was an ancient cultural region in central-southern Mesopotamia (present-day Iraq), with Babylon as its capital. Babylonia emerged as...
, Egypt
Ancient Egypt
Ancient Egypt was an ancient civilization of Northeastern Africa, concentrated along the lower reaches of the Nile River in what is now the modern country of Egypt. Egyptian civilization coalesced around 3150 BC with the political unification of Upper and Lower Egypt under the first pharaoh...
, and especially the Arab
Arab
Arab people, also known as Arabs , are a panethnicity primarily living in the Arab world, which is located in Western Asia and North Africa. They are identified as such on one or more of genealogical, linguistic, or cultural grounds, with tribal affiliations, and intra-tribal relationships playing...
s and Persians
Persian people
The Persian people are part of the Iranian peoples who speak the modern Persian language and closely akin Iranian dialects and languages. The origin of the ethnic Iranian/Persian peoples are traced to the Ancient Iranian peoples, who were part of the ancient Indo-Iranians and themselves part of...
after Islam
Islam
Islam . The most common are and . : Arabic pronunciation varies regionally. The first vowel ranges from ~~. The second vowel ranges from ~~~...
, modern chemistry flourished from the time of Antoine Lavoisier
Antoine Lavoisier
Antoine-Laurent de Lavoisier , the "father of modern chemistry", was a French nobleman prominent in the histories of chemistry and biology...
, who is regarded as the "father of modern chemistry", particularly for his discovery of the law of conservation of mass
Conservation of mass
The law of conservation of mass, also known as the principle of mass/matter conservation, states that the mass of an isolated system will remain constant over time...
, and his refutation of the phlogiston theory
Phlogiston theory
The phlogiston theory , first stated in 1667 by Johann Joachim Becher, is an obsolete scientific theory that postulated the existence of a fire-like element called "phlogiston", which was contained within combustible bodies and released during combustion...
of combustion
Combustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
in 1783. (Phlogiston was supposed to be an imponderable substance liberated by flammable materials in burning.) Mikhail Lomonosov
Mikhail Lomonosov
Mikhail Vasilyevich Lomonosov was a Russian polymath, scientist and writer, who made important contributions to literature, education, and science. Among his discoveries was the atmosphere of Venus. His spheres of science were natural science, chemistry, physics, mineralogy, history, art,...
independently established a tradition of chemistry in Russia
Russia
Russia or , officially known as both Russia and the Russian Federation , is a country in northern Eurasia. It is a federal semi-presidential republic, comprising 83 federal subjects...
in the 18th century. Lomonosov also rejected the phlogiston theory, and anticipated the kinetic theory
Kinetic theory
The kinetic theory of gases describes a gas as a large number of small particles , all of which are in constant, random motion. The rapidly moving particles constantly collide with each other and with the walls of the container...
of gases. He regarded heat as a form of motion, and stated the idea of conservation of matter.
The vitalism debate and organic chemistry
After the nature of combustion (see oxygenOxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
) was settled, another dispute, about vitalism
Vitalism
Vitalism, as defined by the Merriam-Webster dictionary, is#a doctrine that the functions of a living organism are due to a vital principle distinct from biochemical reactions...
and the essential distinction between organic and inorganic substances, was revolutionized by Friedrich Wöhler
Friedrich Wöhler
Friedrich Wöhler was a German chemist, best known for his synthesis of urea, but also the first to isolate several chemical elements.-Biography:He was born in Eschersheim, which belonged to aau...
's accidental synthesis of urea
Urea
Urea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group....
from inorganic substances in 1828. Never before had an organic compound
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
been synthesized from inorganic material. This opened a new research field in chemistry, and by the end of the 19th century, scientists were able to synthesize hundreds of organic compounds. The most important among them are mauve
Mauve
Mauve is a pale lavender-lilac color, one of many in the range of purples. The color mauve is named after the mallow flower....
, magenta
Magenta
Magenta is a color evoked by light stronger in blue and red wavelengths than in yellowish-green wavelengths . In light experiments, magenta can be produced by removing the lime-green wavelengths from white light...
, and other synthetic dye
Dye
A dye is a colored substance that has an affinity to the substrate to which it is being applied. The dye is generally applied in an aqueous solution, and requires a mordant to improve the fastness of the dye on the fiber....
s, as well as the widely used drug aspirin
Aspirin
Aspirin , also known as acetylsalicylic acid , is a salicylate drug, often used as an analgesic to relieve minor aches and pains, as an antipyretic to reduce fever, and as an anti-inflammatory medication. It was discovered by Arthur Eichengrun, a chemist with the German company Bayer...
. The discovery of the artificial synthesis of urea contributed greatly to the theory of isomerism, as the empirical chemical formulas for urea and ammonium
Ammonium
The ammonium cation is a positively charged polyatomic cation with the chemical formula NH. It is formed by the protonation of ammonia...
cyanate
Cyanate
The cyanate ion is an anion with the chemical formula written as [OCN]− or [NCO]−. In aqueous solution it acts as a base, forming isocyanic acid, HNCO. The cyanate ion is an ambidentate ligand, forming complexes with a metal ion in which either the nitrogen or oxygen atom may be the electron-pair...
are identical (see Wöhler synthesis
Wöhler synthesis
rightThe Wöhler synthesis is the conversion of ammonium cyanate into urea. This chemical reaction was discovered in 1828 by Friedrich Wöhler in an attempt to synthesize ammonium cyanate. It is considered the starting point of modern organic chemistry. Although the Wöhler reaction concerns the...
).
Disputes about atomism after Lavoisier

John Dalton
John Dalton FRS was an English chemist, meteorologist and physicist. He is best known for his pioneering work in the development of modern atomic theory, and his research into colour blindness .-Early life:John Dalton was born into a Quaker family at Eaglesfield, near Cockermouth, Cumberland,...
and those who did not, such as Wilhelm Ostwald
Wilhelm Ostwald
Friedrich Wilhelm Ostwald was a Baltic German chemist. He received the Nobel Prize in Chemistry in 1909 for his work on catalysis, chemical equilibria and reaction velocities...
and Ernst Mach
Ernst Mach
Ernst Mach was an Austrian physicist and philosopher, noted for his contributions to physics such as the Mach number and the study of shock waves...
. Although such proponents of the atomic theory as Amedeo Avogadro
Amedeo Avogadro
Lorenzo Romano Amedeo Carlo Avogadro di Quaregna e di Cerreto, Count of Quaregna and Cerreto was an Italian savant. He is most noted for his contributions to molecular theory, including what is known as Avogadro's law...
and Ludwig Boltzmann
Ludwig Boltzmann
Ludwig Eduard Boltzmann was an Austrian physicist famous for his founding contributions in the fields of statistical mechanics and statistical thermodynamics...
made great advances in explaining the behavior of gas
Gas
Gas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
es, this dispute was not finally settled until Jean Perrin's experimental investigation of Einstein
Albert Einstein
Albert Einstein was a German-born theoretical physicist who developed the theory of general relativity, effecting a revolution in physics. For this achievement, Einstein is often regarded as the father of modern physics and one of the most prolific intellects in human history...
's atomic explanation of Brownian motion
Brownian motion
Brownian motion or pedesis is the presumably random drifting of particles suspended in a fluid or the mathematical model used to describe such random movements, which is often called a particle theory.The mathematical model of Brownian motion has several real-world applications...
in the first decade of the 20th century.
Well before the dispute had been settled, many had already applied the concept of atomism to chemistry. A major example was the ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
theory of Svante Arrhenius
Svante Arrhenius
Svante August Arrhenius was a Swedish scientist, originally a physicist, but often referred to as a chemist, and one of the founders of the science of physical chemistry...
which anticipated ideas about atomic substructure that did not fully develop until the 20th century. Michael Faraday
Michael Faraday
Michael Faraday, FRS was an English chemist and physicist who contributed to the fields of electromagnetism and electrochemistry....
was another early worker, whose major contribution to chemistry was electrochemistry
History of electrochemistry
Electrochemistry, a branch of chemistry, went through several changes during its evolution from early principles related to magnets in the early 16th and 17th centuries, to complex theories involving conductivity, electrical charge and mathematical methods. The term electrochemistry was used to...
, in which (among other things) a certain quantity of electricity during electrolysis
Electrolysis
In chemistry and manufacturing, electrolysis is a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction...
or electrodeposition
Underpotential deposition
Underpotential deposition is a phenomenon of electrodeposition of a species at a potential less negative than the equilibrium potential for the reduction of this metal....
of metals was shown to be associated with certain quantities of chemical elements, and fixed quantities of the elements therefore with each other, in specific ratios. These findings, like those of Dalton's combining ratios, were early clues to the atomic nature of matter.
The periodic table
For many decades, the list of known chemical elements had been steadily increasing. A great breakthrough in making sense of this long list (as well as in understanding the internal structure of atoms as discussed below) was Dmitri MendeleevDmitri Mendeleev
Dmitri Ivanovich Mendeleev , was a Russian chemist and inventor. He is credited as being the creator of the first version of the periodic table of elements...
and Lothar Meyer's development of the periodic table
Periodic table
The periodic table of the chemical elements is a tabular display of the 118 known chemical elements organized by selected properties of their atomic structures. Elements are presented by increasing atomic number, the number of protons in an atom's atomic nucleus...
, and particularly Mendeleev's use of it to predict the existence and the properties of germanium
Germanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is a lustrous, hard, grayish-white metalloid in the carbon group, chemically similar to its group neighbors tin and silicon. The isolated element is a semiconductor, with an appearance most similar to elemental silicon....
, gallium
Gallium
Gallium is a chemical element that has the symbol Ga and atomic number 31. Elemental gallium does not occur in nature, but as the gallium salt in trace amounts in bauxite and zinc ores. A soft silvery metallic poor metal, elemental gallium is a brittle solid at low temperatures. As it liquefies...
, and scandium
Scandium
Scandium is a chemical element with symbol Sc and atomic number 21. A silvery-white metallic transition metal, it has historically been sometimes classified as a rare earth element, together with yttrium and the lanthanoids...
, which Mendeleev called ekasilicon, ekaaluminium, and ekaboron
Mendeleev's predicted elements
Professor Dmitri Mendeleev published the first Periodic Table of the Atomic Elements in 1869 based on properties which appeared with some regularity as he laid out the elements from lightest to heaviest....
respectively. Mendeleev made his prediction in 1870; gallium was discovered in 1875, and was found to have roughly the same properties that Mendeleev predicted for it.
The modern definition of chemistry
Classically, before the 20th century, chemistry was defined as the science of the nature of matter and its transformations. It was therefore clearly distinct from physics which was not concerned with such dramatic transformation of matter. Moreover, in contrast to physics, chemistry was not using much of mathematics. Even some were particularly reluctant to using mathematics within chemistry. For example, Auguste ComteAuguste Comte
Isidore Auguste Marie François Xavier Comte , better known as Auguste Comte , was a French philosopher, a founder of the discipline of sociology and of the doctrine of positivism...
wrote in 1830:
However, in the second part of the 19th century, the situation changed and August Kekule wrote in 1867:
After the discovery by Ernest Rutherford
Ernest Rutherford
Ernest Rutherford, 1st Baron Rutherford of Nelson OM, FRS was a New Zealand-born British chemist and physicist who became known as the father of nuclear physics...
and Niels Bohr
Niels Bohr
Niels Henrik David Bohr was a Danish physicist who made foundational contributions to understanding atomic structure and quantum mechanics, for which he received the Nobel Prize in Physics in 1922. Bohr mentored and collaborated with many of the top physicists of the century at his institute in...
of the atomic structure in 1912, and by Marie and Pierre Curie
Pierre Curie
Pierre Curie was a French physicist, a pioneer in crystallography, magnetism, piezoelectricity and radioactivity, and Nobel laureate. He was the son of Dr. Eugène Curie and Sophie-Claire Depouilly Curie ...
of radioactivity, scientists had to change their viewpoint on the nature of matter. The experience acquired by chemists was no longer pertinent to the study of the whole nature of matter but only to aspects related to the electron cloud surrounding the atomic nuclei
Atomic nucleus
The nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911, as a result of Ernest Rutherford's interpretation of the famous 1909 Rutherford experiment performed by Hans Geiger and Ernest Marsden, under the direction of Rutherford. The...
and the movement of the latter in the electric field
Electric field
In physics, an electric field surrounds electrically charged particles and time-varying magnetic fields. The electric field depicts the force exerted on other electrically charged objects by the electrically charged particle the field is surrounding...
induced by the former (see Born-Oppenheimer approximation
Born-Oppenheimer approximation
In quantum chemistry, the computation of the energy and wavefunction of an average-size molecule is a formidable task that is alleviated by the Born–Oppenheimer approximation, named after Max Born and J. Robert Oppenheimer. For instance the benzene molecule consists of 12 nuclei and 42...
). The range of chemistry was thus restricted to the nature of matter around us in conditions which are not too far (or exceptionally far) from standard conditions for temperature and pressure
Standard conditions for temperature and pressure
Standard condition for temperature and pressure are standard sets of conditions for experimental measurements established to allow comparisons to be made between different sets of data...
and in cases where the exposure to radiation is not too different from the natural microwave
Microwave
Microwaves, a subset of radio waves, have wavelengths ranging from as long as one meter to as short as one millimeter, or equivalently, with frequencies between 300 MHz and 300 GHz. This broad definition includes both UHF and EHF , and various sources use different boundaries...
, visible
Visible spectrum
The visible spectrum is the portion of the electromagnetic spectrum that is visible to the human eye. Electromagnetic radiation in this range of wavelengths is called visible light or simply light. A typical human eye will respond to wavelengths from about 390 to 750 nm. In terms of...
or UV radiations on Earth. Chemistry was therefore re-defined as the science of matter that deals with the composition, structure, and properties of substances and with the transformations that they undergo. However the meaning of matter used here relates explicitly to substances made of atoms and molecules, disregarding the matter within the atomic nuclei and its nuclear reaction or matter within highly ionized plasmas. This does not mean that chemistry is never involved with plasma or nuclear sciences or even bosonic fields nowadays, since areas such as Quantum Chemistry and Nuclear Chemistry are currently well developed and formally recognized sub-fields of study under the Chemical sciences (Chemistry), but what is now formally recognized as subject of study under the Chemistry category as a science is always based on the use of concepts that describe or explain phenomena either from matter or to matter in the atomic or molecular scale, including the study of the behavior of many molecules as an aggregate or the study of the effects of a single proton on a single atom, but excluding phenomena that deal with different (more "exotic") types of matter (e.g. Bose-Einstein condensate, Higgs Boson, dark matter, naked singularity, etc.) and excluding principles that refer to intrinsic abstract laws of nature in which their concepts can be formulated completely without a precise formal molecular or atomic paradigmatic view (e.g. Quantum Chromodynamics, Quantum Electrodynamics, String Theory, parts of Cosmology (see Cosmochemistry
Cosmochemistry
Cosmochemistry or chemical cosmology is the study of the chemical composition of matter in the universe and the processes that led to those compositions. This is done primarily through the study of the chemical composition of meteorites and other physical samples...
), certain areas of Nuclear Physics (see Nuclear Chemistry
Nuclear chemistry
Nuclear chemistry is the subfield of chemistry dealing with radioactivity, nuclear processes and nuclear properties.It is the chemistry of radioactive elements such as the actinides, radium and radon together with the chemistry associated with equipment which are designed to perform nuclear...
),etc.). Nevertheless the field of chemistry is still, on our human scale, very broad and the claim that chemistry is everywhere is accurate.
Quantum chemistry
Some view the birth of quantum chemistry in the discovery of the Schrödinger equationSchrödinger equation
The Schrödinger equation was formulated in 1926 by Austrian physicist Erwin Schrödinger. Used in physics , it is an equation that describes how the quantum state of a physical system changes in time....
and its application to the hydrogen atom
Hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively-charged proton and a single negatively-charged electron bound to the nucleus by the Coulomb force...
in 1926. However, the 1927 article of Walter Heitler
Walter Heitler
Walter Heinrich Heitler was a German physicist who made contributions to quantum electrodynamics and quantum field theory...
and Fritz London
Fritz London
Fritz Wolfgang London was a German theoretical physicist. His fundamental contributions to the theories of chemical bonding and of intermolecular forces are today considered classic and are discussed in standard textbooks of physical chemistry.With his brother Heinz, he made a significant...
is often recognised as the first milestone in the history of quantum chemistry. This is the first application of quantum mechanics
Quantum mechanics
Quantum mechanics, also known as quantum physics or quantum theory, is a branch of physics providing a mathematical description of much of the dual particle-like and wave-like behavior and interactions of energy and matter. It departs from classical mechanics primarily at the atomic and subatomic...
to the diatomic hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
molecule, and thus to the phenomenon of the chemical bond
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
. In the following years much progress was accomplished by Edward Teller
Edward Teller
Edward Teller was a Hungarian-American theoretical physicist, known colloquially as "the father of the hydrogen bomb," even though he did not care for the title. Teller made numerous contributions to nuclear and molecular physics, spectroscopy , and surface physics...
, Robert S. Mulliken
Robert S. Mulliken
Robert Sanderson Mulliken was an American physicist and chemist, primarily responsible for the early development of molecular orbital theory, i.e. the elaboration of the molecular orbital method of computing the structure of molecules. Dr. Mulliken received the Nobel Prize for chemistry in 1966...
, Max Born
Max Born
Max Born was a German-born physicist and mathematician who was instrumental in the development of quantum mechanics. He also made contributions to solid-state physics and optics and supervised the work of a number of notable physicists in the 1920s and 30s...
, J. Robert Oppenheimer, Linus Pauling
Linus Pauling
Linus Carl Pauling was an American chemist, biochemist, peace activist, author, and educator. He was one of the most influential chemists in history and ranks among the most important scientists of the 20th century...
, Erich Hückel
Erich Hückel
Erich Armand Arthur Joseph Hückel was a German physicist and physical chemist. He is known for two major contributions:*The Debye–Hückel theory of electrolytic solutions...
, Douglas Hartree
Douglas Hartree
Douglas Rayner Hartree PhD, FRS was an English mathematician and physicist most famous for the development of numerical analysis and its application to the Hartree-Fock equations of atomic physics and the construction of the meccano differential analyser.-Early life:Douglas Hartree was born in...
, Vladimir Aleksandrovich Fock, to cite a few.
Still, skepticism remained as to the general power of quantum mechanics applied to complex chemical systems. The situation around 1930 is described by Paul Dirac
Paul Dirac
Paul Adrien Maurice Dirac, OM, FRS was an English theoretical physicist who made fundamental contributions to the early development of both quantum mechanics and quantum electrodynamics...
:
Hence the quantum mechanical methods developed in the 1930s and 1940s are often referred to as theoretical molecular
Molecular physics
Molecular physics is the study of the physical properties of molecules, the chemical bonds between atoms as well as the molecular dynamics. Its most important experimental techniques are the various types of spectroscopy...
or atomic physics
Atomic physics
Atomic physics is the field of physics that studies atoms as an isolated system of electrons and an atomic nucleus. It is primarily concerned with the arrangement of electrons around the nucleus and...
to underline the fact that they were more the application of quantum mechanics to chemistry and spectroscopy
Spectroscopy
Spectroscopy is the study of the interaction between matter and radiated energy. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, e.g., by a prism. Later the concept was expanded greatly to comprise any interaction with radiative...
than answers to chemically relevant questions.
In the 1940s many physicists turned from molecular
Molecular physics
Molecular physics is the study of the physical properties of molecules, the chemical bonds between atoms as well as the molecular dynamics. Its most important experimental techniques are the various types of spectroscopy...
or atomic physics
Atomic physics
Atomic physics is the field of physics that studies atoms as an isolated system of electrons and an atomic nucleus. It is primarily concerned with the arrangement of electrons around the nucleus and...
to nuclear physics
Nuclear physics
Nuclear physics is the field of physics that studies the building blocks and interactions of atomic nuclei. The most commonly known applications of nuclear physics are nuclear power generation and nuclear weapons technology, but the research has provided application in many fields, including those...
(like J. Robert Oppenheimer or Edward Teller
Edward Teller
Edward Teller was a Hungarian-American theoretical physicist, known colloquially as "the father of the hydrogen bomb," even though he did not care for the title. Teller made numerous contributions to nuclear and molecular physics, spectroscopy , and surface physics...
). In 1951, a milestone article in quantum chemistry is the seminal paper of Clemens C. J. Roothaan
Clemens C. J. Roothaan
Clemens C.J. Roothaan was born in 1918 in Nijmegen, the Netherlands. He enrolled TU Delft in 1935 to study electrical engineering. During World War II he was first detained as a prisoner of war camp, later he was sent for a year to a concentration camp in Vught...
on Roothaan equations
Roothaan equations
The Roothaan equations are a representation of the Hartree-Fock equation in a non orthonormal basis set which can be of Gaussian-type or Slater-type. It applies to closed-shell molecules or atoms where all molecular orbitals or atomic orbitals, respectively, are doubly occupied. This is generally...
. It opened the avenue to the solution of the self-consistent field equations for small molecules like hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
or nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
. Those computations were performed with the help of tables of integrals which were computed on the most advanced computers of the time.
Molecular biology and biochemistry
By the mid 20th century, in principle, the integration of physics and chemistry was extensive, with chemical properties explained as the result of the electronElectron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
ic structure of the atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
; Linus Pauling
Linus Pauling
Linus Carl Pauling was an American chemist, biochemist, peace activist, author, and educator. He was one of the most influential chemists in history and ranks among the most important scientists of the 20th century...
's book on The Nature of the Chemical Bond used the principles of quantum mechanics to deduce bond angles in ever-more complicated molecules. However, though some principles deduced from quantum mechanics were able to predict qualitatively some chemical features for biologically relevant molecules, they were, till the end of the 20th century, more a collection of rules, observations, and recipes than rigorous ab initio
Ab initio
ab initio is a Latin term used in English, meaning from the beginning.ab initio may also refer to:* Ab Initio , a leading ETL Tool Software Company in the field of Data Warehousing.* ab initio quantum chemistry methods...
quantitative methods.

James D. Watson
James Dewey Watson is an American molecular biologist, geneticist, and zoologist, best known as one of the co-discoverers of the structure of DNA in 1953 with Francis Crick...
and Francis Crick
Francis Crick
Francis Harry Compton Crick OM FRS was an English molecular biologist, biophysicist, and neuroscientist, and most noted for being one of two co-discoverers of the structure of the DNA molecule in 1953, together with James D. Watson...
deduced the double helical structure of DNA
DNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
by constructing models constrained by and informed by the knowledge of the chemistry of the constituent parts and the X-ray diffraction patterns obtained by Rosalind Franklin
Rosalind Franklin
Rosalind Elsie Franklin was a British biophysicist and X-ray crystallographer who made critical contributions to the understanding of the fine molecular structures of DNA, RNA, viruses, coal and graphite...
. This discovery lead to an explosion of research into the biochemistry
Biochemistry
Biochemistry, sometimes called biological chemistry, is the study of chemical processes in living organisms, including, but not limited to, living matter. Biochemistry governs all living organisms and living processes...
of life.
In the same year, the Miller-Urey experiment
Miller-Urey experiment
The Miller and Urey experiment was an experiment that simulated hypothetical conditions thought at the time to be present on the early Earth, and tested for the occurrence of chemical origins of life. Specifically, the experiment tested Alexander Oparin's and J. B. S...
demonstrated that basic constituents of protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
, simple amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s, could themselves be built up from simpler molecules in a simulation
Simulation
Simulation is the imitation of some real thing available, state of affairs, or process. The act of simulating something generally entails representing certain key characteristics or behaviours of a selected physical or abstract system....
of primordial processes
Process (science)
In science, a process is every sequence of changes of a real object/body which is observable using the scientific method. Therefore, all sciences analyze and model processes....
on Earth. Though many questions remain about the true nature of the origin of life, this was the first attempt by chemists to study hypothetical processes in the laboratory under controlled conditions.
In 1983 Kary Mullis
Kary Mullis
Kary Banks Mullis is a Nobel Prize winning American biochemist, author, and lecturer. In recognition of his improvement of the polymerase chain reaction technique, he shared the 1993 Nobel Prize in Chemistry with Michael Smith and earned the Japan Prize in the same year. The process was first...
devised a method for the in-vitro amplification of DNA, known as the polymerase chain reaction
Polymerase chain reaction
The polymerase chain reaction is a scientific technique in molecular biology to amplify a single or a few copies of a piece of DNA across several orders of magnitude, generating thousands to millions of copies of a particular DNA sequence....
(PCR), which revolutionized the chemical processes used in the laboratory to manipulate it. PCR could be used to synthesize specific pieces of DNA and made possible the sequencing of DNA
DNA sequencing
DNA sequencing includes several methods and technologies that are used for determining the order of the nucleotide bases—adenine, guanine, cytosine, and thymine—in a molecule of DNA....
of organisms, which culminated in the huge human genome project
Human Genome Project
The Human Genome Project is an international scientific research project with a primary goal of determining the sequence of chemical base pairs which make up DNA, and of identifying and mapping the approximately 20,000–25,000 genes of the human genome from both a physical and functional...
.
An important piece in the double helix puzzle was solved by one of Pauling's student Matthew Meselson
Matthew Meselson
Matthew Stanley Meselson is an American geneticist and molecular biologist whose research was important in showing how DNA replicates, recombines and is repaired in cells. In his mature years, he has been an active chemical and biological weapons activist and consultant...
and Frank Stahl, the result of their collaboration (Meselson-Stahl experiment
Meselson-Stahl experiment
The Meselson–Stahl experiment was an experiment by Matthew Meselson and Franklin Stahl in 1958 which supported the hypothesis that DNA replication was semiconservative. Semiconservative replication means that when the double stranded DNA helix was replicated, each of the two double stranded DNA...
) has been called as "the most beautiful experiment in biology".
They used a centrifugation technique that sorted molecules according to differences in weight. Because nitrogen atoms are a component of DNA, they were labelled and therefore tracked in replication in bacteria.
Chemical industry
The later part of the nineteenth century saw a huge increase in the exploitation of petroleumPetroleum
Petroleum or crude oil is a naturally occurring, flammable liquid consisting of a complex mixture of hydrocarbons of various molecular weights and other liquid organic compounds, that are found in geologic formations beneath the Earth's surface. Petroleum is recovered mostly through oil drilling...
extracted from the earth for the production of a host of chemicals and largely replaced the use of whale oil
Whale oil
Whale oil is the oil obtained from the blubber of various species of whales, particularly the three species of right whale and the bowhead whale prior to the modern era, as well as several other species of baleen whale...
, coal tar
Coal tar
Coal tar is a brown or black liquid of extremely high viscosity, which smells of naphthalene and aromatic hydrocarbons. Coal tar is among the by-products when coal iscarbonized to make coke or gasified to make coal gas...
and naval stores
Naval stores
Naval Stores is a broad term which originally applied to the resin-based components used in building and maintaining wooden sailing ships, a category which includes cordage, mask, turpentine, rosin, pitch and tar...
used previously. Large scale production and refinement of petroleum
Oil refinery
An oil refinery or petroleum refinery is an industrial process plant where crude oil is processed and refined into more useful petroleum products, such as gasoline, diesel fuel, asphalt base, heating oil, kerosene, and liquefied petroleum gas...
provided feedstocks for liquid fuels
Liquid fuels
Liquid fuels are those combustible or energy-generating molecules that can be harnessed to create mechanical energy, usually producing kinetic energy; they also must take the shape of their container...
such as gasoline
Gasoline
Gasoline , or petrol , is a toxic, translucent, petroleum-derived liquid that is primarily used as a fuel in internal combustion engines. It consists mostly of organic compounds obtained by the fractional distillation of petroleum, enhanced with a variety of additives. Some gasolines also contain...
and diesel, solvents, lubricants, asphalt
Asphalt
Asphalt or , also known as bitumen, is a sticky, black and highly viscous liquid or semi-solid that is present in most crude petroleums and in some natural deposits, it is a substance classed as a pitch...
, waxes, and for the production of many of the common materials of the modern world, such as synthetic fibers, plastic
Plastic
A plastic material is any of a wide range of synthetic or semi-synthetic organic solids used in the manufacture of industrial products. Plastics are typically polymers of high molecular mass, and may contain other substances to improve performance and/or reduce production costs...
s, paints, detergent
Detergent
A detergent is a surfactant or a mixture of surfactants with "cleaning properties in dilute solutions." In common usage, "detergent" refers to alkylbenzenesulfonates, a family of compounds that are similar to soap but are less affected by hard water...
s, pharmaceuticals, adhesives and ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
as fertilizer
Fertilizer
Fertilizer is any organic or inorganic material of natural or synthetic origin that is added to a soil to supply one or more plant nutrients essential to the growth of plants. A recent assessment found that about 40 to 60% of crop yields are attributable to commercial fertilizer use...
and for other uses. Many of these required new catalysts and the utilization of chemical engineering
Chemical engineering
Chemical engineering is the branch of engineering that deals with physical science , and life sciences with mathematics and economics, to the process of converting raw materials or chemicals into more useful or valuable forms...
for their cost-effective production.
In the mid-twentieth century, control of the electronic structure of semiconductor
Semiconductor
A semiconductor is a material with electrical conductivity due to electron flow intermediate in magnitude between that of a conductor and an insulator. This means a conductivity roughly in the range of 103 to 10−8 siemens per centimeter...
materials was made precise by the creation of large ingots of extremely pure single crystals of silicon
Silicon
Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table...
and germanium
Germanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is a lustrous, hard, grayish-white metalloid in the carbon group, chemically similar to its group neighbors tin and silicon. The isolated element is a semiconductor, with an appearance most similar to elemental silicon....
. Accurate control of their chemical composition by doping with other elements made the production of the solid state transistor
Transistor
A transistor is a semiconductor device used to amplify and switch electronic signals and power. It is composed of a semiconductor material with at least three terminals for connection to an external circuit. A voltage or current applied to one pair of the transistor's terminals changes the current...
in 1951 and made possible the production of tiny integrated circuit
Integrated circuit
An integrated circuit or monolithic integrated circuit is an electronic circuit manufactured by the patterned diffusion of trace elements into the surface of a thin substrate of semiconductor material...
s for use in electronic devices, especially computers.
Histories and timelines
- Atomic theoryAtomic theoryIn chemistry and physics, atomic theory is a theory of the nature of matter, which states that matter is composed of discrete units called atoms, as opposed to the obsolete notion that matter could be divided into any arbitrarily small quantity...
- CupellationCupellationCupellation is a metallurgical process in which ores or alloyed metals are treated under high temperatures and carefully controlled operations in order to separate noble metals, like gold and silver, from base metals like lead, copper, zinc, arsenic, antimony or bismuth, that might be present in...
- History of chromatographyHistory of chromatographyThe history of chromatography spans from the mid-19th century to the 21st. Chromatography, literally "color writing", was used—and named— in the first decade of the 20th century, primarily for the separation of plant pigments such as chlorophyll and carotenoids...
- History of electrochemistryHistory of electrochemistryElectrochemistry, a branch of chemistry, went through several changes during its evolution from early principles related to magnets in the early 16th and 17th centuries, to complex theories involving conductivity, electrical charge and mathematical methods. The term electrochemistry was used to...
- History of the moleculeHistory of the moleculeIn chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms....
- History of molecular biologyHistory of molecular biologyThe history of molecular biology begins in the 1930s with the convergence of various, previously distinct biological disciplines: biochemistry, genetics, microbiology, and virology...
- History of the periodic tableHistory of the periodic tableThe history of the periodic table reflects over a century of growth in the understanding of chemical properties, and culminates with the publication of the first actual periodic table by Dmitri Mendeleev in 1869...
- History of physicsHistory of physicsAs forms of science historically developed out of philosophy, physics was originally referred to as natural philosophy, a term describing a field of study concerned with "the workings of nature".-Early history:...
- History of science and technologyHistory of science and technologyThe history of science and technology is a field of history which examines how humanity's understanding of the natural world and ability to manipulate it have changed over the centuries...
- History of thermodynamicsHistory of thermodynamicsThe history of thermodynamics is a fundamental strand in the history of physics, the history of chemistry, and the history of science in general...
- History of energyHistory of energyThe word energy derives from Greek ἐνέργεια , which appears for the first time in the work Nicomachean Ethics of Aristotle in the 4th century BCE....
- History of molecular theory
- History of materials scienceHistory of materials scienceThe history of materials science is the study of how different materials were used as influenced by the history of Earth and the culture of the peoples of the Earth.-Prehistory:...
- Timeline of chemical elements discoveries
- Timeline of chemistryTimeline of chemistryThe timeline of chemistry lists important works, discoveries, ideas, inventions, and experiments that significantly changed humanity's understanding of the modern science known as chemistry, defined as the scientific study of the composition of matter and of its interactions...
- Timeline of materials technologyTimeline of materials technologyMajor innovations in materials technology-BC:* 29,000–25,000 BC – First pottery appears*3rd millennium BC – Copper metallurgy is invented and copper is used for ornamentation*2nd millennium BC – Bronze is used for weapons and armour...
- Timeline of atomic and subatomic physics
- Timeline of thermodynamics, statistical mechanics, and random processesTimeline of thermodynamics, statistical mechanics, and random processesA timeline of events related to thermodynamics.- Before 1800 :* 1650 – Otto von Guericke builds the first vacuum pump* 1660 – Robert Boyle experimentally discovers Boyle's Law, relating the pressure and volume of a gas...
- List of years in science
- Nobel Prize in chemistryNobel Prize in ChemistryThe Nobel Prize in Chemistry is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outstanding contributions in chemistry, physics, literature,...
- The Chemical History of a CandleThe Chemical History of a CandleThe Chemical History of a Candle was the title of a series of six lectures on the chemistry and physics of flames given by Michael Faraday at the Royal Institution...
Chemists
listed chronologically:- List of chemists
- Joseph BlackJoseph BlackJoseph Black FRSE FRCPE FPSG was a Scottish physician and chemist, known for his discoveries of latent heat, specific heat, and carbon dioxide. He was professor of Medicine at University of Glasgow . James Watt, who was appointed as philosophical instrument maker at the same university...
, 1728–1799 - Joseph PriestleyJoseph PriestleyJoseph Priestley, FRS was an 18th-century English theologian, Dissenting clergyman, natural philosopher, chemist, educator, and political theorist who published over 150 works...
, 1733–1804 - Carl Wilhelm ScheeleCarl Wilhelm ScheeleCarl Wilhelm Scheele was a German-Swedish pharmaceutical chemist. Isaac Asimov called him "hard-luck Scheele" because he made a number of chemical discoveries before others who are generally given the credit...
, 1742–1786 - Alessandro VoltaAlessandro VoltaCount Alessandro Giuseppe Antonio Anastasio Gerolamo Umberto Volta was a Lombard physicist known especially for the invention of the battery in 1800.-Early life and works:...
, 1745–1827 - Jacques CharlesJacques CharlesJacques Alexandre César Charles was a French inventor, scientist, mathematician, and balloonist.Charles and the Robert brothers launched the world's first hydrogen-filled balloon in August 1783, then in December 1783, Charles and his co-pilot Nicolas-Louis Robert ascended to a height of about...
, 1746–1823 - Claude Louis BertholletClaude Louis BertholletClaude Louis Berthollet was a Savoyard-French chemist who became vice president of the French Senate in 1804.-Biography:...
, 1748–1822 - Joseph-Louis Gay-Lussac, 1778–1850
- Humphry DavyHumphry DavySir Humphry Davy, 1st Baronet FRS MRIA was a British chemist and inventor. He is probably best remembered today for his discoveries of several alkali and alkaline earth metals, as well as contributions to the discoveries of the elemental nature of chlorine and iodine...
, 1778–1829 - Jöns Jakob BerzeliusJöns Jakob BerzeliusJöns Jacob Berzelius was a Swedish chemist. He worked out the modern technique of chemical formula notation, and is together with John Dalton, Antoine Lavoisier, and Robert Boyle considered a father of modern chemistry...
, inventor of modern chemical notation, 1779–1848 - Justus von LiebigJustus von LiebigJustus von Liebig was a German chemist who made major contributions to agricultural and biological chemistry, and worked on the organization of organic chemistry. As a professor, he devised the modern laboratory-oriented teaching method, and for such innovations, he is regarded as one of the...
, 1803–1873 - Louis PasteurLouis PasteurLouis Pasteur was a French chemist and microbiologist born in Dole. He is remembered for his remarkable breakthroughs in the causes and preventions of diseases. His discoveries reduced mortality from puerperal fever, and he created the first vaccine for rabies and anthrax. His experiments...
, 1822–1895 - Stanislao CannizzaroStanislao CannizzaroStanislao Cannizzaro, FRS was an Italian chemist. He is remembered today largely for the Cannizzaro reaction and for his influential role in the atomic-weight deliberations of the Karlsruhe Congress in 1860.-Biography:...
, 1826–1910 - Friedrich August Kekulé von StradonitzFriedrich August Kekulé von StradonitzFriedrich August Kekule von Stradonitz was a German organic chemist. From the 1850s until his death, Kekule was one of the most prominent chemists in Europe, especially in theoretical chemistry...
, 1829–1896 - Willard Gibbs, 1839–1903
- J. H. van 't Hoff, 1852–1911
- Marie CurieMarie CurieMarie Skłodowska-Curie was a physicist and chemist famous for her pioneering research on radioactivity. She was the first person honored with two Nobel Prizes—in physics and chemistry...
, 1867–1934 - Victor GrignardVictor GrignardFrançois Auguste Victor Grignard was a Nobel Prize-winning French chemist.Grignard was the son of a sail maker. After studying mathematics at Lyon he transferred to chemistry and discovered the synthetic reaction bearing his name in 1900...
, 1871–1935 - Gilbert N. LewisGilbert N. LewisGilbert Newton Lewis was an American physical chemist known for the discovery of the covalent bond , his purification of heavy water, his reformulation of chemical thermodynamics in a mathematically rigorous manner accessible to ordinary chemists, his theory of Lewis acids and...
, 1875–1946 - Otto HahnOtto HahnOtto Hahn FRS was a German chemist and Nobel laureate, a pioneer in the fields of radioactivity and radiochemistry. He is regarded as "the father of nuclear chemistry". Hahn was a courageous opposer of Jewish persecution by the Nazis and after World War II he became a passionate campaigner...
, 1879–1968 - Irving LangmuirIrving LangmuirIrving Langmuir was an American chemist and physicist. His most noted publication was the famous 1919 article "The Arrangement of Electrons in Atoms and Molecules" in which, building on Gilbert N. Lewis's cubical atom theory and Walther Kossel's chemical bonding theory, he outlined his...
, 1881–1957 - Walther NernstWalther NernstWalther Hermann Nernst FRS was a German physical chemist and physicist who is known for his theories behind the calculation of chemical affinity as embodied in the third law of thermodynamics, for which he won the 1920 Nobel Prize in chemistry...
, 1864–1941 - Linus PaulingLinus PaulingLinus Carl Pauling was an American chemist, biochemist, peace activist, author, and educator. He was one of the most influential chemists in history and ranks among the most important scientists of the 20th century...
, 1901–1994 - Glenn T. SeaborgGlenn T. SeaborgGlenn Theodore Seaborg was an American scientist who won the 1951 Nobel Prize in Chemistry for "discoveries in the chemistry of the transuranium elements", contributed to the discovery and isolation of ten elements, and developed the actinide concept, which led to the current arrangement of the...
, 1912–1999 - Frederick SangerFrederick SangerFrederick Sanger, OM, CH, CBE, FRS is an English biochemist and a two-time Nobel laureate in chemistry, the only person to have been so. In 1958 he was awarded a Nobel prize in chemistry "for his work on the structure of proteins, especially that of insulin"...
, 1918- - Rudolph A. MarcusRudolph A. MarcusRudolph "Rudy" Arthur Marcus is a Canadian-born chemist who received the 1992 Nobel Prize in Chemistry for his theory of electron transfer. Marcus theory, named after him, provides a thermodynamic and kinetic framework for describing one electron outer-sphere electron transfer.He was born in...
, 1923- - Harold KrotoHarold KrotoSir Harold Walter Kroto, FRS , born Harold Walter Krotoschiner, is a British chemist and one of the three recipients to share the 1996 Nobel Prize in Chemistry with Robert Curl and Richard Smalley....
, 1939- - Richard SmalleyRichard SmalleyRichard Errett Smalley was the Gene and Norman Hackerman Professor of Chemistry and a Professor of Physics and Astronomy at Rice University, in Houston, Texas...
, 1943–2005 - Peter AtkinsPeter AtkinsPeter William Atkins is a British chemist and former Professor of Chemistry at the University of Oxford and a Fellow of Lincoln College. He is a prolific writer of popular chemistry textbooks, including Physical Chemistry, Inorganic Chemistry, and Molecular Quantum Mechanics...
, 1940-
Further reading
- Servos, John W.John ServosJohn William Servos is an American professor and historian of science. His research centers on the historical development of science as a discourse and in the form of institutions and on how science has situated itself historically in the culture at large.Servos is the Anson D...
, Physical chemistry from Ostwald to Pauling : the making of a science in America, Princeton, N.J. : Princeton University Press, 1990. ISBN 0691085668
Documentaries
- BBCBBCThe British Broadcasting Corporation is a British public service broadcaster. Its headquarters is at Broadcasting House in the City of Westminster, London. It is the largest broadcaster in the world, with about 23,000 staff...
(2010). Chemistry: A Volatile HistoryChemistry: A Volatile HistoryChemistry: A Volatile History is a 2010 BBC documentary on the history of chemistry presented by Jim Al-Khalili. It was nominated for the 2010 British Academy Television Awards in the category Specialist Factual.-Introduction:...
.
External links
- ChemisLab - Chemists of the Past
- SHAC: Society for the History of Alchemy and Chemistry

