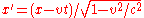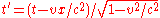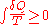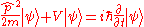List of laws in science
Encyclopedia
The laws of science are various established scientific law
s, or physical law
s as they are sometimes called, that are considered universal and invariable facts of the physical universe
. Laws of science may, however, be disproved if new facts or evidence contradicts them. A "law" differs from hypotheses, theories
, postulates, principle
s, etc., in that a law is an analytic statement, usually with an empirically determined constant. A theory may contain a set of laws, or a theory may be implied from an empirically determined law.
Laws of electromagnetism and gravitation
Laws of energy
Laws of heat transfer
Gas laws
These fundamental laws follow from homogeneity of space
, time
and phase
(see Emmy Noether theorem).
They are low-limit solutions to relativity
. Alternative formulations of Newtonian mechanics are Lagrangian
and Hamiltonian
mechanics. Euler's laws of motion are extensions of Newton's laws.
Fluid dynamics
Scientific law
A scientific law is a statement that explains what something does in science just like Newton's law of universal gravitation. A scientific law must always apply under the same conditions, and implies a causal relationship between its elements. The law must be confirmed and broadly agreed upon...
s, or physical law
Physical law
A physical law or scientific law is "a theoretical principle deduced from particular facts, applicable to a defined group or class of phenomena, and expressible by the statement that a particular phenomenon always occurs if certain conditions be present." Physical laws are typically conclusions...
s as they are sometimes called, that are considered universal and invariable facts of the physical universe
Universe
The Universe is commonly defined as the totality of everything that exists, including all matter and energy, the planets, stars, galaxies, and the contents of intergalactic space. Definitions and usage vary and similar terms include the cosmos, the world and nature...
. Laws of science may, however, be disproved if new facts or evidence contradicts them. A "law" differs from hypotheses, theories
Scientific theory
A scientific theory comprises a collection of concepts, including abstractions of observable phenomena expressed as quantifiable properties, together with rules that express relationships between observations of such concepts...
, postulates, principle
Principle
A principle is a law or rule that has to be, or usually is to be followed, or can be desirably followed, or is an inevitable consequence of something, such as the laws observed in nature or the way that a system is constructed...
s, etc., in that a law is an analytic statement, usually with an empirically determined constant. A theory may contain a set of laws, or a theory may be implied from an empirically determined law.
Incomplete list of scientific laws
Laws of motion- Kepler’s three laws of planetary motionKepler's laws of planetary motionIn astronomy, Kepler's laws give a description of the motion of planets around the Sun.Kepler's laws are:#The orbit of every planet is an ellipse with the Sun at one of the two foci....
- Newton’s three laws of motionNewton's laws of motionNewton's laws of motion are three physical laws that form the basis for classical mechanics. They describe the relationship between the forces acting on a body and its motion due to those forces...
- Euler's lawsEuler's lawsEuler's laws of motion, formulated by Leonhard Euler about 50 years after Isaac Newton formulated his laws about the motion of particles, extends them to rigid body motion.-Euler's first law:...
of rigid bodyRigid bodyIn physics, a rigid body is an idealization of a solid body of finite size in which deformation is neglected. In other words, the distance between any two given points of a rigid body remains constant in time regardless of external forces exerted on it...
motion
Laws of electromagnetism and gravitation
- Gauss's lawGauss's lawIn physics, Gauss's law, also known as Gauss's flux theorem, is a law relating the distribution of electric charge to the resulting electric field. Gauss's law states that:...
or Coulomb's LawCoulomb's lawCoulomb's law or Coulomb's inverse-square law, is a law of physics describing the electrostatic interaction between electrically charged particles. It was first published in 1785 by French physicist Charles Augustin de Coulomb and was essential to the development of the theory of electromagnetism...
of electrostatics - Gauss's law for magnetism
- Ampère's circuital law
- Faraday's law of inductionFaraday's law of inductionFaraday's law of induction dates from the 1830s, and is a basic law of electromagnetism relating to the operating principles of transformers, inductors, and many types of electrical motors and generators...
- Lorentz forceLorentz forceIn physics, the Lorentz force is the force on a point charge due to electromagnetic fields. It is given by the following equation in terms of the electric and magnetic fields:...
Law - Joule's laws (first law)
- Ohm's lawOhm's lawOhm's law states that the current through a conductor between two points is directly proportional to the potential difference across the two points...
- Kirchhoff's circuit lawsKirchhoff's circuit lawsKirchhoff's circuit laws are two equalities that deal with the conservation of charge and energy in electrical circuits, and were first described in 1845 by Gustav Kirchhoff...
- Biot–Savart law of electromagnetism
- Lenz's lawLenz's lawLenz's law is a common way of understanding how electromagnetic circuits must always obey Newton's third law and The Law of Conservation of Energy...
- Newton’s law of universal gravitation
Laws of energy
- Laws of thermodynamicsLaws of thermodynamicsThe four laws of thermodynamics summarize its most important facts. They define fundamental physical quantities, such as temperature, energy, and entropy, in order to describe thermodynamic systems. They also describe the transfer of energy as heat and work in thermodynamic processes...
Laws of heat transfer
- Fourier's law of heat conduction
- Newton’s law of cooling
- Laws of thermal radiation
- Planck's law
- Stefan–Boltzmann law
- Wien's displacement lawWien's displacement lawWien's displacement law states that the wavelength distribution of thermal radiation from a black body at any temperature has essentially the same shape as the distribution at any other temperature, except that each wavelength is displaced on the graph...
- Kirchhoff's law of thermal radiationKirchhoff's law of thermal radiationIn thermodynamics, Kirchhoff's law of thermal radiation, or Kirchhoff's law for short, is a general statement equating emission and absorption in heated objects, proposed by Gustav Kirchhoff in 1859, following from general considerations of thermodynamic equilibrium and detailed balance.An object...
Gas laws
-
- Boyle’s lawBoyle's lawBoyle's law is one of many gas laws and a special case of the ideal gas law. Boyle's law describes the inversely proportional relationship between the absolute pressure and volume of a gas, if the temperature is kept constant within a closed system...
- Charles's lawCharles's lawCharles' law is an experimental gas law which describes how gases tend to expand when heated. It was first published by French natural philosopher Joseph Louis Gay-Lussac in 1802, although he credited the discovery to unpublished work from the 1780s by Jacques Charles...
- Gay-Lussac's lawGay-Lussac's lawThe expression Gay-Lussac's law is used for each of the two relationships named after the French chemist Joseph Louis Gay-Lussac and which concern the properties of gases, though it is more usually applied to his law of combining volumes, the first listed here...
- Combined gas lawCombined gas lawThe combined gas law is a gas law which combines Charles's law, Boyle's law, and Gay-Lussac's law. These laws each relate one thermodynamic variable to another mathematically while holding everything else constant. Charles's law states that volume and temperature are directly proportional to each...
- Avogadro's lawAvogadro's lawAvogadro's law is a gas law named after Amedeo Avogadro who, in 1811, hypothesized that two given samples of an ideal gas, at the same temperature, pressure and volume, contain the same number of molecules...
- Ideal gas lawIdeal gas lawThe ideal gas law is the equation of state of a hypothetical ideal gas. It is a good approximation to the behavior of many gases under many conditions, although it has several limitations. It was first stated by Émile Clapeyron in 1834 as a combination of Boyle's law and Charles's law...
- Graham's lawGraham's lawGraham's law, known as Graham's law of effusion, was formulated by Scottish physical chemist Thomas Graham in 1846. Graham found experimentally that the rate of effusion of a gas is inversely proportional to the square root of the mass of its particles...
of effusion - Fick's laws of diffusion
- Dalton's lawDalton's lawIn chemistry and physics, Dalton's law states that the total pressure exerted by a gaseous mixture is equal to the sum of the partial pressures of each individual component in a gas mixture...
of partial pressures - Henry's lawHenry's lawIn physics, Henry's law is one of the gas laws formulated by William Henry in 1803. It states that:An equivalent way of stating the law is that the solubility of a gas in a liquid at a particular temperature is proportional to the pressure of that gas above the liquid...
of dissolved gas - Joule's laws (Joule's second law of ideal gas )
- Boyle’s law
Conservation laws
Most significant laws in science are conservation laws:- Conservation of massConservation of massThe law of conservation of mass, also known as the principle of mass/matter conservation, states that the mass of an isolated system will remain constant over time...
law - Conservation of energyConservation of energyThe nineteenth century law of conservation of energy is a law of physics. It states that the total amount of energy in an isolated system remains constant over time. The total energy is said to be conserved over time...
law - Conservation of momentumMomentumIn classical mechanics, linear momentum or translational momentum is the product of the mass and velocity of an object...
law - Conservation of angular momentumAngular momentumIn physics, angular momentum, moment of momentum, or rotational momentum is a conserved vector quantity that can be used to describe the overall state of a physical system...
law - Charge conservationCharge conservationIn physics, charge conservation is the principle that electric charge can neither be created nor destroyed. The net quantity of electric charge, the amount of positive charge minus the amount of negative charge in the universe, is always conserved...
law
These fundamental laws follow from homogeneity of space
Space
Space is the boundless, three-dimensional extent in which objects and events occur and have relative position and direction. Physical space is often conceived in three linear dimensions, although modern physicists usually consider it, with time, to be part of a boundless four-dimensional continuum...
, time
Time
Time is a part of the measuring system used to sequence events, to compare the durations of events and the intervals between them, and to quantify rates of change such as the motions of objects....
and phase
Phase (waves)
Phase in waves is the fraction of a wave cycle which has elapsed relative to an arbitrary point.-Formula:The phase of an oscillation or wave refers to a sinusoidal function such as the following:...
(see Emmy Noether theorem).
Relativity
- Special relativitySpecial relativitySpecial relativity is the physical theory of measurement in an inertial frame of reference proposed in 1905 by Albert Einstein in the paper "On the Electrodynamics of Moving Bodies".It generalizes Galileo's...
- Constancy of the speed of lightSpeed of lightThe speed of light in vacuum, usually denoted by c, is a physical constant important in many areas of physics. Its value is 299,792,458 metres per second, a figure that is exact since the length of the metre is defined from this constant and the international standard for time...
- Lorentz transformations – Transformations of Cartesian coordinatesCartesian coordinate systemA Cartesian coordinate system specifies each point uniquely in a plane by a pair of numerical coordinates, which are the signed distances from the point to two fixed perpendicular directed lines, measured in the same unit of length...
between relatively moving reference frameFrame of referenceA frame of reference in physics, may refer to a coordinate system or set of axes within which to measure the position, orientation, and other properties of objects in it, or it may refer to an observational reference frame tied to the state of motion of an observer.It may also refer to both an...
s. -
- Mass-energy equivalence
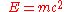 (energyEnergyIn physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
(energyEnergyIn physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
= massMassMass can be defined as a quantitive measure of the resistance an object has to change in its velocity.In physics, mass commonly refers to any of the following three properties of matter, which have been shown experimentally to be equivalent:...
× speed of lightSpeed of lightThe speed of light in vacuum, usually denoted by c, is a physical constant important in many areas of physics. Its value is 299,792,458 metres per second, a figure that is exact since the length of the metre is defined from this constant and the international standard for time...
2)
- Constancy of the speed of light
- General relativityGeneral relativityGeneral relativity or the general theory of relativity is the geometric theory of gravitation published by Albert Einstein in 1916. It is the current description of gravitation in modern physics...
- Energy-momentum (including mass via E=mc2) curves spacetime.
- This is described by the Einstein field equationsEinstein field equationsThe Einstein field equations or Einstein's equations are a set of ten equations in Albert Einstein's general theory of relativity which describe the fundamental interaction of gravitation as a result of spacetime being curved by matter and energy...
: -
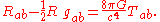
-
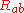 is the Ricci tensor,
is the Ricci tensor,  is the Ricci scalar,
is the Ricci scalar, 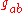 is the metric tensorMetric tensor (general relativity)In general relativity, the metric tensor is the fundamental object of study. It may loosely be thought of as a generalization of the gravitational field familiar from Newtonian gravitation...
is the metric tensorMetric tensor (general relativity)In general relativity, the metric tensor is the fundamental object of study. It may loosely be thought of as a generalization of the gravitational field familiar from Newtonian gravitation...
,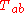 is the stress-energy tensorStress-energy tensorThe stress–energy tensor is a tensor quantity in physics that describes the density and flux of energy and momentum in spacetime, generalizing the stress tensor of Newtonian physics. It is an attribute of matter, radiation, and non-gravitational force fields...
is the stress-energy tensorStress-energy tensorThe stress–energy tensor is a tensor quantity in physics that describes the density and flux of energy and momentum in spacetime, generalizing the stress tensor of Newtonian physics. It is an attribute of matter, radiation, and non-gravitational force fields...
, and the constant is given in terms of (piPi' is a mathematical constant that is the ratio of any circle's circumference to its diameter. is approximately equal to 3.14. Many formulae in mathematics, science, and engineering involve , which makes it one of the most important mathematical constants...
(piPi' is a mathematical constant that is the ratio of any circle's circumference to its diameter. is approximately equal to 3.14. Many formulae in mathematics, science, and engineering involve , which makes it one of the most important mathematical constants...
), (the speed of lightSpeed of lightThe speed of light in vacuum, usually denoted by c, is a physical constant important in many areas of physics. Its value is 299,792,458 metres per second, a figure that is exact since the length of the metre is defined from this constant and the international standard for time...
(the speed of lightSpeed of lightThe speed of light in vacuum, usually denoted by c, is a physical constant important in many areas of physics. Its value is 299,792,458 metres per second, a figure that is exact since the length of the metre is defined from this constant and the international standard for time...
) and (the gravitational constantGravitational constantThe gravitational constant, denoted G, is an empirical physical constant involved in the calculation of the gravitational attraction between objects with mass. It appears in Newton's law of universal gravitation and in Einstein's theory of general relativity. It is also known as the universal...
(the gravitational constantGravitational constantThe gravitational constant, denoted G, is an empirical physical constant involved in the calculation of the gravitational attraction between objects with mass. It appears in Newton's law of universal gravitation and in Einstein's theory of general relativity. It is also known as the universal...
). 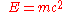 where
where 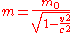
- This is described by the Einstein field equations
- Energy-momentum (including mass via E=mc2) curves spacetime.
Laws of classical mechanics
Newton's laws of motionNewton's laws of motion
Newton's laws of motion are three physical laws that form the basis for classical mechanics. They describe the relationship between the forces acting on a body and its motion due to those forces...
They are low-limit solutions to relativity
Theory of relativity
The theory of relativity, or simply relativity, encompasses two theories of Albert Einstein: special relativity and general relativity. However, the word relativity is sometimes used in reference to Galilean invariance....
. Alternative formulations of Newtonian mechanics are Lagrangian
Lagrangian mechanics
Lagrangian mechanics is a re-formulation of classical mechanics that combines conservation of momentum with conservation of energy. It was introduced by the Italian-French mathematician Joseph-Louis Lagrange in 1788....
and Hamiltonian
Hamiltonian mechanics
Hamiltonian mechanics is a reformulation of classical mechanics that was introduced in 1833 by Irish mathematician William Rowan Hamilton.It arose from Lagrangian mechanics, a previous reformulation of classical mechanics introduced by Joseph Louis Lagrange in 1788, but can be formulated without...
mechanics. Euler's laws of motion are extensions of Newton's laws.
-
- Law of inertiaInertiaInertia is the resistance of any physical object to a change in its state of motion or rest, or the tendency of an object to resist any change in its motion. It is proportional to an object's mass. The principle of inertia is one of the fundamental principles of classical physics which are used to...
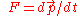 . When the mass is constant, this implies
. When the mass is constant, this implies  .
. . Force of a on b equals the negative force of b on a, or for every actionAction (physics)In physics, action is an attribute of the dynamics of a physical system. It is a mathematical functional which takes the trajectory, also called path or history, of the system as its argument and has a real number as its result. Action has the dimension of energy × time, and its unit is...
. Force of a on b equals the negative force of b on a, or for every actionAction (physics)In physics, action is an attribute of the dynamics of a physical system. It is a mathematical functional which takes the trajectory, also called path or history, of the system as its argument and has a real number as its result. Action has the dimension of energy × time, and its unit is...
there is an equal but opposite reactionReaction (physics)The third of Newton's laws of motion of classical mechanics states that forces always occur in pairs. Every action is accompanied by a reaction of equal magnitude but opposite direction. This principle is commonly known in the Latin language as actio et reactio. The attribution of which of the two...
.
- Law of inertia
Fluid dynamics
Fluid dynamics
In physics, fluid dynamics is a sub-discipline of fluid mechanics that deals with fluid flow—the natural science of fluids in motion. It has several subdisciplines itself, including aerodynamics and hydrodynamics...
- Navier–Stokes equations
-
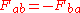
- Poiseuille's law (voluminal laminar stationary flow of incompressiblePotential flowIn fluid dynamics, potential flow describes the velocity field as the gradient of a scalar function: the velocity potential. As a result, a potential flow is characterized by an irrotational velocity field, which is a valid approximation for several applications...
uniform viscousViscosityViscosity is a measure of the resistance of a fluid which is being deformed by either shear or tensile stress. In everyday terms , viscosity is "thickness" or "internal friction". Thus, water is "thin", having a lower viscosity, while honey is "thick", having a higher viscosity...
liquid through a cylindrical tube with the constant circular cross-section)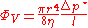
Other
- Newton-Euler lawAngular momentumIn physics, angular momentum, moment of momentum, or rotational momentum is a conserved vector quantity that can be used to describe the overall state of a physical system...
(rotation of rigid bodies)
Laws of gravitation
Classical laws
- Laws of Kepler (planetary motion)
- General law of gravitation – gravitational force between two objects equals the gravitational constantGravitational constantThe gravitational constant, denoted G, is an empirical physical constant involved in the calculation of the gravitational attraction between objects with mass. It appears in Newton's law of universal gravitation and in Einstein's theory of general relativity. It is also known as the universal...
times the product of the massMassMass can be defined as a quantitive measure of the resistance an object has to change in its velocity.In physics, mass commonly refers to any of the following three properties of matter, which have been shown experimentally to be equivalent:...
es divided by the distance between them squared. Newton's second law was gravity's effect on us humans. 
- This law is really just the low limit solution of Einstein's field equations and is not accurate with modern high precision gravitational measurements.
Modern laws: see General Relativity above.
Electromagnetic laws
Pre-Maxwell laws
- Coulomb's lawCoulomb's lawCoulomb's law or Coulomb's inverse-square law, is a law of physics describing the electrostatic interaction between electrically charged particles. It was first published in 1785 by French physicist Charles Augustin de Coulomb and was essential to the development of the theory of electromagnetism...
– ForceForceIn physics, a force is any influence that causes an object to undergo a change in speed, a change in direction, or a change in shape. In other words, a force is that which can cause an object with mass to change its velocity , i.e., to accelerate, or which can cause a flexible object to deform...
between any two chargesElectric chargeElectric charge is a physical property of matter that causes it to experience a force when near other electrically charged matter. Electric charge comes in two types, called positive and negative. Two positively charged substances, or objects, experience a mutual repulsive force, as do two...
is equal to the product of the charges divided by 4 piPi' is a mathematical constant that is the ratio of any circle's circumference to its diameter. is approximately equal to 3.14. Many formulae in mathematics, science, and engineering involve , which makes it one of the most important mathematical constants...
times the vacuum permittivity times the distanceDistanceDistance is a numerical description of how far apart objects are. In physics or everyday discussion, distance may refer to a physical length, or an estimation based on other criteria . In mathematics, a distance function or metric is a generalization of the concept of physical distance...
squared between the two charges.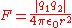
- Ohm's lawOhm's lawOhm's law states that the current through a conductor between two points is directly proportional to the potential difference across the two points...
- Kirchhoff's circuit lawsKirchhoff's circuit lawsKirchhoff's circuit laws are two equalities that deal with the conservation of charge and energy in electrical circuits, and were first described in 1845 by Gustav Kirchhoff...
(current and voltageVoltageVoltage, otherwise known as electrical potential difference or electric tension is the difference in electric potential between two points — or the difference in electric potential energy per unit charge between two points...
laws) - Kirchhoff's law of thermal radiationKirchhoff's law of thermal radiationIn thermodynamics, Kirchhoff's law of thermal radiation, or Kirchhoff's law for short, is a general statement equating emission and absorption in heated objects, proposed by Gustav Kirchhoff in 1859, following from general considerations of thermodynamic equilibrium and detailed balance.An object...
Maxwell's equationsMaxwell's equationsMaxwell's equations are a set of partial differential equations that, together with the Lorentz force law, form the foundation of classical electrodynamics, classical optics, and electric circuits. These fields in turn underlie modern electrical and communications technologies.Maxwell's equations...
ElectricElectric fieldIn physics, an electric field surrounds electrically charged particles and time-varying magnetic fields. The electric field depicts the force exerted on other electrically charged objects by the electrically charged particle the field is surrounding...
and magnetic fieldMagnetic fieldA magnetic field is a mathematical description of the magnetic influence of electric currents and magnetic materials. The magnetic field at any given point is specified by both a direction and a magnitude ; as such it is a vector field.Technically, a magnetic field is a pseudo vector;...
s unified:
Name Partial differential form Gauss's law Gauss's lawIn physics, Gauss's law, also known as Gauss's flux theorem, is a law relating the distribution of electric charge to the resulting electric field. Gauss's law states that:...
:
Gauss's law for magnetism: 
Faraday's law of induction Faraday's law of inductionFaraday's law of induction dates from the 1830s, and is a basic law of electromagnetism relating to the operating principles of transformers, inductors, and many types of electrical motors and generators...
: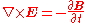
Ampère's law Ampère's lawIn classical electromagnetism, Ampère's circuital law, discovered by André-Marie Ampère in 1826, relates the integrated magnetic field around a closed loop to the electric current passing through the loop...
+ Maxwell's extension: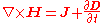
Thermodynamic laws
Laws of ThermodynamicsThermodynamicsThermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
- Zeroth law of thermodynamicsZeroth law of thermodynamicsThe zeroth law of thermodynamics is a generalization principle of thermal equilibrium among bodies, or thermodynamic systems, in contact.The zeroth law states that if two systems are in thermal equilibrium with a third system, they are also in thermal equilibrium with each other.Systems are said to...
- If two systems are in thermal equilibriumThermal equilibriumThermal equilibrium is a theoretical physical concept, used especially in theoretical texts, that means that all temperatures of interest are unchanging in time and uniform in space...
with a third system, then they are in thermal equilibrium with one another.
- If two systems are in thermal equilibrium
- First law of thermodynamicsFirst law of thermodynamicsThe first law of thermodynamics is an expression of the principle of conservation of work.The law states that energy can be transformed, i.e. changed from one form to another, but cannot be created nor destroyed...
- The change in energy dU in a system is accounted for entirely by the heat δQ absorbed by the system and the work δW done by the system:
- The change in energy dU in a system is accounted for entirely by the heat δQ absorbed by the system and the work δW done by the system:
- Second law of thermodynamicsSecond law of thermodynamicsThe second law of thermodynamics is an expression of the tendency that over time, differences in temperature, pressure, and chemical potential equilibrate in an isolated physical system. From the state of thermodynamic equilibrium, the law deduced the principle of the increase of entropy and...
- Third law of thermodynamicsThird law of thermodynamicsThe third law of thermodynamics is a statistical law of nature regarding entropy:For other materials, the residual entropy is not necessarily zero, although it is always zero for a perfect crystal in which there is only one possible ground state.-History:...
- As the temperature T of a system approaches absolute zero, the entropy S approaches a minimum value C: as T → 0, S → C.
- Onsager reciprocal relationsOnsager reciprocal relationsIn thermodynamics, the Onsager reciprocal relations express the equality of certain ratios between flows and forces in thermodynamic systems out of equilibrium, but where a notion of local equilibrium exists....
– sometimes called the Fourth Law of Thermodynamics-
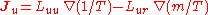 ;
; -
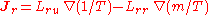 .
.
-
Other
- Newton's law of heat conduction
- Fourier's law
Quantum laws
Quantum mechanicsQuantum mechanicsQuantum mechanics, also known as quantum physics or quantum theory, is a branch of physics providing a mathematical description of much of the dual particle-like and wave-like behavior and interactions of energy and matter. It departs from classical mechanics primarily at the atomic and subatomic...
- Planck–Einstein lawPlanck constantThe Planck constant , also called Planck's constant, is a physical constant reflecting the sizes of energy quanta in quantum mechanics. It is named after Max Planck, one of the founders of quantum theory, who discovered it in 1899...
for the energyEnergyIn physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
of photonPhotonIn physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force...
s – Energy equals Planck's constant multiplied by the frequencyFrequencyFrequency is the number of occurrences of a repeating event per unit time. It is also referred to as temporal frequency.The period is the duration of one cycle in a repeating event, so the period is the reciprocal of the frequency...
of the lightLightLight or visible light is electromagnetic radiation that is visible to the human eye, and is responsible for the sense of sight. Visible light has wavelength in a range from about 380 nanometres to about 740 nm, with a frequency range of about 405 THz to 790 THz...
.
-

- Heisenberg uncertainty principle – UncertaintyUncertaintyUncertainty is a term used in subtly different ways in a number of fields, including physics, philosophy, statistics, economics, finance, insurance, psychology, sociology, engineering, and information science...
in position multiplied by uncertainty in momentumMomentumIn classical mechanics, linear momentum or translational momentum is the product of the mass and velocity of an object...
is equal to or greater than the reduced Planck constant divided by 2. -
- Matter wavelength – Laid the foundations of particle-wave duality and was the key idea in the Schrödinger equation.
- Schrödinger equationSchrödinger equationThe Schrödinger equation was formulated in 1926 by Austrian physicist Erwin Schrödinger. Used in physics , it is an equation that describes how the quantum state of a physical system changes in time....
– Describes the time dependence of a quantum mechanical system. -
- Heisenberg uncertainty principle – Uncertainty
- or more compactly
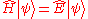
-
- The HamiltonianHamiltonianHamiltonian may refer toIn mathematics :* Hamiltonian system* Hamiltonian path, in graph theory** Hamiltonian cycle, a special case of a Hamiltonian path* Hamiltonian group, in group theory* Hamiltonian...
(in quantum mechanics) H is a self-adjoint operatorSelf-adjoint operatorIn mathematics, on a finite-dimensional inner product space, a self-adjoint operator is an operator that is its own adjoint, or, equivalently, one whose matrix is Hermitian, where a Hermitian matrix is one which is equal to its own conjugate transpose...
acting on the state space,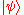 is the instantaneous quantum state vector at time t, position r, i is the unit imaginary numberImaginary numberAn imaginary number is any number whose square is a real number less than zero. When any real number is squared, the result is never negative, but the square of an imaginary number is always negative...
is the instantaneous quantum state vector at time t, position r, i is the unit imaginary numberImaginary numberAn imaginary number is any number whose square is a real number less than zero. When any real number is squared, the result is never negative, but the square of an imaginary number is always negative...
,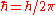 is the reduced Planck's constant. Note that
is the reduced Planck's constant. Note that 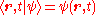 , see Dirac notation.
, see Dirac notation.
- The Hamiltonian
-
It is thought that the successful integration of Einstein's field equations with the uncertainty principleUncertainty principleIn quantum mechanics, the Heisenberg uncertainty principle states a fundamental limit on the accuracy with which certain pairs of physical properties of a particle, such as position and momentum, can be simultaneously known...
and Schrödinger equationSchrödinger equationThe Schrödinger equation was formulated in 1926 by Austrian physicist Erwin Schrödinger. Used in physics , it is an equation that describes how the quantum state of a physical system changes in time....
, something no one has achieved so far with a testable theoryTheoryThe English word theory was derived from a technical term in Ancient Greek philosophy. The word theoria, , meant "a looking at, viewing, beholding", and referring to contemplation or speculation, as opposed to action...
, will lead to a theory of quantum gravityQuantum gravityQuantum gravity is the field of theoretical physics which attempts to develop scientific models that unify quantum mechanics with general relativity...
, the most basic physical law sought after today.
Radiation
EM Radiation, LightRadiationIn physics, radiation is a process in which energetic particles or energetic waves travel through a medium or space. There are two distinct types of radiation; ionizing and non-ionizing...
laws
- Planck's law of black body radiationPlanck's law of black body radiationIn physics, Planck's law describes the amount of energy emitted by a black body in radiation of a certain wavelength . The law is named after Max Planck, who originally proposed it in 1900. The law was the first to accurately describe black body radiation, and resolved the ultraviolet catastrophe...
(spectral densitySpectral densityIn statistical signal processing and physics, the spectral density, power spectral density , or energy spectral density , is a positive real function of a frequency variable associated with a stationary stochastic process, or a deterministic function of time, which has dimensions of power per hertz...
in a radiationRadiationIn physics, radiation is a process in which energetic particles or energetic waves travel through a medium or space. There are two distinct types of radiation; ionizing and non-ionizing...
of a black-body) - Wien's law (wavelength of the peak of the emission of a black body) :λ0T = kw
- Stefan-Boltzmann law (total radiation from a black body)
-
- Beer-LambertBeer-Lambert lawIn optics, the Beer–Lambert law, also known as Beer's law or the Lambert–Beer law or the Beer–Lambert–Bouguer law relates the absorption of light to the properties of the material through which the light is travelling.-Equations:The law states that there is a logarithmic dependence between the...
(light absorption)
- Radioactive decayRadioactive decayRadioactive decay is the process by which an atomic nucleus of an unstable atom loses energy by emitting ionizing particles . The emission is spontaneous, in that the atom decays without any physical interaction with another particle from outside the atom...
law (number of atoms in a radionuclide)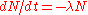
Laws of chemistry and matter
Chemical laws are those laws of nature relevant to chemistryChemistryChemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
. The most fundamental concept in chemistry is the law of conservation of mass, which states that there is no detectable change in the quantity of matter during an ordinary chemical reactionChemical reactionA chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
. Modern physics shows that it is actually energyEnergyIn physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
that is conserved, and that energy and mass are related; a concept which becomes important in nuclear chemistryNuclear chemistryNuclear chemistry is the subfield of chemistry dealing with radioactivity, nuclear processes and nuclear properties.It is the chemistry of radioactive elements such as the actinides, radium and radon together with the chemistry associated with equipment which are designed to perform nuclear...
. Conservation of energyConservation of energyThe nineteenth century law of conservation of energy is a law of physics. It states that the total amount of energy in an isolated system remains constant over time. The total energy is said to be conserved over time...
leads to the important concepts of equilibriumChemical equilibriumIn a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
, thermodynamicsThermodynamicsThermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
, and kineticsChemical kineticsChemical kinetics, also known as reaction kinetics, is the study of rates of chemical processes. Chemical kinetics includes investigations of how different experimental conditions can influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition...
.
Additional laws of chemistry elaborate on the law of conservation of mass. Joseph ProustJoseph ProustJoseph Louis Proust was a French chemist.-Life:Joseph L. Proust was born on September 26, 1754 in Angers, France. His father served as an apothecary in Angers. Joseph studied chemistry in his father’s shop and later came to Paris where he gained the appointment of apothecary in chief to the...
's law of definite composition says that pure chemicals are composed of elements in a definite formulation; we now know that the structural arrangement of these elements is also important.
DaltonJohn DaltonJohn Dalton FRS was an English chemist, meteorologist and physicist. He is best known for his pioneering work in the development of modern atomic theory, and his research into colour blindness .-Early life:John Dalton was born into a Quaker family at Eaglesfield, near Cockermouth, Cumberland,...
's law of multiple proportionsLaw of multiple proportionsIn chemistry, the law of multiple proportions is one of the basic laws of stoichiometry, alongside the law of definite proportions. It is sometimes called Dalton's Law after its discoverer, the English chemist John Dalton.The statement of the law is:...
says that these chemicals will present themselves in proportions that are small whole numbers (i.e. 1:2 O:H in water); although in many systems (notably biomacromolecules and minerals) the ratios tend to require large numbers, and are frequently represented as a fraction.
More modern laws of chemistry define the relationship between energy and transformations.
- In equilibrium, molecules exist in mixture defined by the transformations possible on the timescale of the equilibrium, and are in a ratio defined by the intrinsic energy of the molecules—the lower the intrinsic energy, the more abundant the molecule.
- Transforming one structure to another requires the input of energy to cross an energy barrier; this can come from the intrinsic energy of the molecules themselves, or from an external source which will generally accelerate transformations. The higher the energy barrier, the slower the transformation occurs.
- There is a hypothetical intermediate, or transition structure, that corresponds to the structure at the top of the energy barrier. The Hammond–Leffler postulateHammond's PostulateHammond's postulate, also referred to as the Hammond–Leffler postulate, is a hypothesis, derived from transition state theory, concerning the transition state of organic chemical reactions, which states that:-Interpreting the postulate:...
states that this structure looks most similar to the product or starting material which has intrinsic energy closest to that of the energy barrier. Stabilizing this hypothetical intermediate through chemical interaction is one way to achieve catalysisCatalysisCatalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
. - All chemical processes are reversible (law of microscopic reversibilityMicroscopic reversibilityThe principle of Microscopic reversibility in physics and chemistry is twofold:* First, it states that the microscopic detailed dynamics of particles and fields is time-reversible because the microscopic equations of motion are symmetric with respect to inversion in time ;* Second, it relates to...
) although some processes have such an energy bias, they are essentially irreversible. - Avogadro's lawAvogadro's lawAvogadro's law is a gas law named after Amedeo Avogadro who, in 1811, hypothesized that two given samples of an ideal gas, at the same temperature, pressure and volume, contain the same number of molecules...
(Equal volumes of ideal or perfect gases, at the same temperature and pressure, contain the same number of particles, or molecules.) - Dulong–Petit lawDulong–Petit lawThe Dulong–Petit law, a chemical law proposed in 1819 by French physicists Pierre Louis Dulong and Alexis Thérèse Petit, states the classical expression for the molar specific heat capacity of a crystal...
(specific heat capacity at constant volume)
Gas lawsGas lawsThe early gas laws were developed at the end of the 18th century, when scientists began to realize that relationships between the pressure, volume and temperature of a sample of gas could be obtained which would hold for all gases...
Other less significant (non fundamental) laws are the mathematical consequences of the above conservation laws for derivative physical quantities (mathematically defined as forceForceIn physics, a force is any influence that causes an object to undergo a change in speed, a change in direction, or a change in shape. In other words, a force is that which can cause an object with mass to change its velocity , i.e., to accelerate, or which can cause a flexible object to deform...
, pressurePressurePressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
, temperatureTemperatureTemperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
, densityDensityThe mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ . In some cases , density is also defined as its weight per unit volume; although, this quantity is more properly called specific weight...
, force fields, etc.):
- Boyle's lawBoyle's lawBoyle's law is one of many gas laws and a special case of the ideal gas law. Boyle's law describes the inversely proportional relationship between the absolute pressure and volume of a gas, if the temperature is kept constant within a closed system...
(pressurePressurePressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
and volumeVolumeVolume is the quantity of three-dimensional space enclosed by some closed boundary, for example, the space that a substance or shape occupies or contains....
of ideal gasIdeal gasAn ideal gas is a theoretical gas composed of a set of randomly-moving, non-interacting point particles. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics.At normal conditions such as...
) - Charles and Gay-Lussac (gases expand equally with the same change of temperatureTemperatureTemperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
) - Ideal gas lawIdeal gas lawThe ideal gas law is the equation of state of a hypothetical ideal gas. It is a good approximation to the behavior of many gases under many conditions, although it has several limitations. It was first stated by Émile Clapeyron in 1834 as a combination of Boyle's law and Charles's law...

Other laws
- Buys-Ballot's lawBuys-Ballot's lawIn meteorology, Buys Ballot's law may be expressed as follows: In the Northern Hemisphere, if a person stands with his back to the wind, the low pressure area will be on his left. This is because wind travels counterclockwise around low pressure zones in the Northern Hemisphere...
(wind travels counterclockwise around low pressure systems in the Northern HemisphereNorthern HemisphereThe Northern Hemisphere is the half of a planet that is north of its equator—the word hemisphere literally means “half sphere”. It is also that half of the celestial sphere north of the celestial equator...
)
See also
- List of laws
- Physical lawPhysical lawA physical law or scientific law is "a theoretical principle deduced from particular facts, applicable to a defined group or class of phenomena, and expressible by the statement that a particular phenomenon always occurs if certain conditions be present." Physical laws are typically conclusions...
– includes discussion of what constitutes a law - List of scientific laws named after people
External Links
- Physics Formulary, a useful book in different formats containing many or the physical laws and formulae.
- Eformulae.com, website containing most of the formulae in different disciplines.
- Kirchhoff's circuit laws
- Poiseuille's law (voluminal laminar stationary flow of incompressible