
Nuclear fuel and reactor accidents
Encyclopedia
This page is devoted to a discussion of how uranium dioxide
nuclear fuel
behaves during both normal nuclear reactor
operation and under reactor accident conditions such as overheating. Work in this area is often very expensive to conduct, and so has often been performed on a collaborative basis between groups of countries, usually under the aegis of the CSNI.

gas to permit better thermal contact between the fuel and the cladding. During use the amount of gas inside the fuel pin can increase because of the formation of noble gas
es (krypton
and xenon
) by the fission process. If a Loss-of-coolant accident (LOCA) (e.g. Three Mile Island
) or a Reactivity Initiated Accident (RIA) (e.g. Chernobyl
or SL-1
) occurs then the temperature of this gas can increase. As the fuel pin is sealed the pressure
of the gas will increase (PV = nRT) and it is possible to deform and burst the cladding. It has been noticed that both corrosion
and irradiation
can alter the properties of the zirconium alloy
commonly used as cladding, making it brittle
. As a result the experiments using unirradated zirconium alloy tubes can be misleading.
According to one paper the following difference between the cladding failure mode of unused and used fuel was seen.
Unirradiated fuel rods were pressurized before being placed in a special reactor at the Japanese Nuclear Safety Research Reactor
(NSRR) where they were subjected to a simulated RIA transient. These rods failed after ballooning late in the transient when the cladding temperature was high. The failure of the cladding in these tests was ductile, and it was a burst opening.
The used fuel (61 GW days/tonne
of uranium) failed early in the transient with a brittle fracture which was a longitudinal crack.
If was found that hydride
d zirconium tube is weaker and the bursting pressure is lower.
The common failure process of fuel in the water-cooled reactors is a transition to film boiling and subsequent ignition of zirconium cladding in the steam. The effects of the intense hot hydrogen reaction product flow on the fuel pellets and on the bundle's wall well represented on the sidebar picture.
can swell during use, this is because of effects such as bubble formation in the fuel and the damage which occurs to the lattice of the solid. The swelling of the fuel can impose mechanical stresses upon the cladding which covers the fuel. A document on the subject of the swelling of the fuel can be downloaded from the NASA
web site.
may become free. For example see.
A report on the release of 85Kr, 106Ru and 137Cs from uranium when air is present has been written. It was found that uranium dioxide was converted to U3O8 between about 300 and 500 °C in air. They report that this process requires some time to start, after the induction time the sample gains mass. The authors report that a layer of U3O7 was present on the uranium dioxide surface during this induction time. They report that 3 to 8% of the krypton
-85 was released, and that much less of the ruthenium
(0.5%) and caesium
(2.6 x 10−3%) occurred during the oxidation of the uranium dioxide.
), if a power surge occurs as a result of a reactivity initiated accident, an understanding of the transfer of heat from the surface of the cladding to the water is very useful. In a French study, metal pipe immersed in water (both under typical PWR
and pond conditions), was electrically heated to simulate the generation of heat within a fuel pin by nuclear processes. The temperature
of the pipe was monitored by thermocouple
s and for the tests conducted under PWR
conditions the water entering the larger pipe (14.2 mm diameter) holding the test metal pipe (9.5 mm outside diameter and 600 mm long) was at 280 oC and 15 MPa. The water was flowing past the inner pipe at circa 4 ms−1 and the cladding was subjected to heating at 2200 to 4900 °C s−1 to simulate an RIA. It was found that as the temperature of the cladding increased the rate of heat transfer from the surface of the cladding increased at first as the water boiled at nucleation
sites. When the heat flux is greater than the critical heat flux
a boiling crisis occurs. This occurs as the temperature of the fuel cladding surface increases so that the surface of the metal was too hot (surface dries out) for nucleation boiling. When the surface dries out the rate of heat transfer
decreases, after a further increase in the temperature of the metal surface the boiling resumes but it is now film boiling.
alloys can undergo stress corrosion cracking
when exposed to iodine, the iodine
is formed as a fission product
which depending on the nature of the fuel can escape from the pellet. It has been shown that iodine causes the rate of cracking in pressurised zircaloy
-4 tubing to increase.
cooled graphite
moderated reactors such as magnox
and AGR
power reactors an important corrosion
reaction is the reaction of a molecule
of carbon dioxide
with graphite (carbon
) to form two molecules of carbon monoxide
. This is one of the processes which limits the working life of this type of reactor.
on the water (radiolysis
) forms hydrogen peroxide
and oxygen
. These can cause stress corrosion cracking
of metal parts which include fuel
cladding and other pipework. To mitigate this hydrazine
and hydrogen
are injected into a BWR or PWR
primary cooling circuit as corrosion inhibitor
s to adjust the redox
properties of the system. A review of recent developments on this topic has been published.
for some time before water is reintroduced into the reactor to cool the fuel. During this time when the hot cladding is exposed to steam some oxidation of the zirconium
will occur to form a zirconium oxide which is more zirconium rich than zirconia. This Zr(O) phase is the α-phase, further oxidation forms zirconia. The longer the cladding is exposed to steam the less ductile it will be. One measure of the ductility is to compress a ring along a diameter (at a constant rate of displacement, in this case 2 mm min−1) until the first crack occurs, then the ring will start to fail. The elongation which occurs between when the maximum force is applied and when the mechanical load is declined to 80% of the load required to induce the first crack is the L0.8 value in mm. The more ductile a sample is the greater this L0.8 value will be.
In one experiment the zirconium is heated in steam to 1473 K, the sample is slowly cooled in steam to 1173 K before being quenched in water. As the heating time at 1473 K is increased the zirconium becomes more brittle and the L0.8 value declines.
causes the properties of steels to become poorer, for instance SS316
becomes less ductile and less tough
. Also creep
and stress corrosion cracking
become worse. Papers on this effect continue to be published.
, the core of the pellet expands more than the rim. Because of the thermal stress thus formed the fuel cracks, the cracks tend to go from the center to the edge in a star shaped pattern. A PhD thesis on the subject has been published by a student at the Royal Institute of Technology
in Stockholm
(Sweden
).
The cracking of the fuel has an effect on the release of radioactivity from fuel both under accident conditions and also when the spent fuel is used as the final disposal form. The cracking increases the surface area of the fuel which increases the rate at which fission products can leave the fuel.
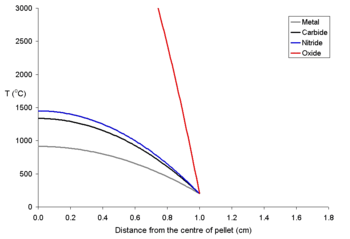
The temperature of the fuel varies as a function of the distance from the center to the rim. At distance x from the center the temperature (Tx) is described by the equation
where ρ is the power density (W m−3) and Kf is the thermal conductivity
.
Tx = TRim + ρ (rpellet² – x²) (4 Kf)−1
To explain this a for a series of fuel pellets being used with a rim temperature of 200 °C (typical for a BWR) with different diameters and power densities of 250 Wm−3 have been modeled using the above equation. These fuel pellets are rather large; it is normal to use oxide pellets which are about 10 mm in diameter.
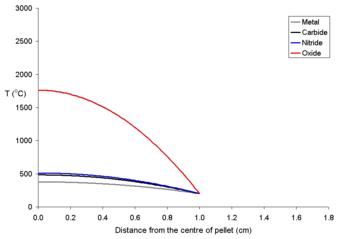
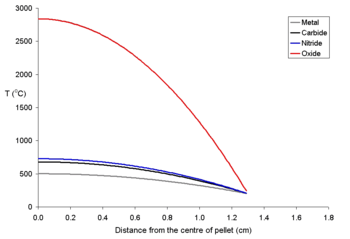
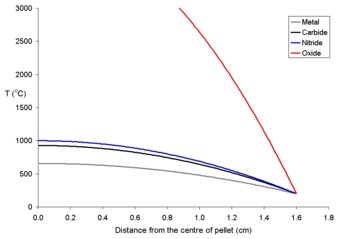 To show the effects of different power densities on the centerline temperatures two graphs for 20 mm pellets at different power levels are shown below. It is clear that for all pellets (and most true of uranium dioxide) that for a given sized pellet that a limit must be set on the power density
To show the effects of different power densities on the centerline temperatures two graphs for 20 mm pellets at different power levels are shown below. It is clear that for all pellets (and most true of uranium dioxide) that for a given sized pellet that a limit must be set on the power density
. It is likely that the maths used for these calculations would be used to explain how electrical fuses
function and also it could be used to predict the centerline temperature in any system where heat is released throughout a cylinder shaped object.
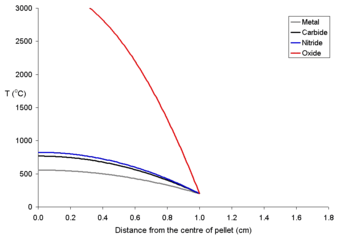
tubes holding the pellet are broken then a greater release of radioactive caesium from the fuel will occur. It is important to understand that the 134Cs and 137Cs are formed in different ways, and hence as a result the two caesium isotopes can be found at different parts of a fuel pin.
It is clear that the volatile iodine
and xenon
isotopes have minutes in which they can diffuse out of the pellet and into the gap between the fuel and the cladding. Here the xenon can decay to the long lived caesium isotope.
These fission yields were calculated for 235U assuming thermal neutrons (0.0253 eV) using data from the chart of the nuclides.
as 134Xe is a stable isotope. As a result of this different mode of formation the physical location of 134Cs can differ from that of 137Cs.
These fission yields were calculated for 235U assuming thermal neutrons (0.0253 eV) using data from the chart of the nuclides.
The fuels varied in their ability to retain the fission xenon
; the first of the three fuels retained 97% of the 133Xe, the second retained 94% while the last fuel only retained 76% of this xenon isotope. The 133Xe is a long-lived radioactive isotope which can diffuse slowly out of the pellet before being neutron activated
to form 134Cs. The more short-lived 137Xe was less able to leach out of the pellets; 99%, 98% and 95% of the 137Xe was retained within the pellets. It was also found that the 137Cs
concentration in the core of the pellet was much lower than the concentration in the rim of the pellet, while the less volatile 106Ru was spread more evenly throughout the pellets.
The following fuel is particles of solid solution
of urania in yttria-stabilized zirconia
dispersed in alumina which had burnt up
to 105 GW-days per cubic meter. The scanning electron microscope
(SEM) is of the interface between the alumina and a fuel particle. It can be seen that the fission products are well confined to within the fuel, little of the fission products have entered the alumina matrix. The neodymium
is spread throughout the fuel in a uniform manner, while the caesium
is almost homogenously spread out throughout the fuel. The caesium concentration is slightly higher at two points where xenon bubbles are present. Much of the xenon is present in bubbles, while almost all of the ruthenium
is present in the form of nanoparticle
s. The ruthenium nanoparticles are not always colocated with the xenon bubbles.
a recently SCRAM
ed core was starved of cooling water, as a result of the decay heat
the core dried out and the fuel was damaged. Attempts were made to recool the core using water. According to the International Atomic Energy Agency
for a 3,000 MW(t) PWR
the normal coolant radioactivity levels are shown below in the table, and the coolant activities for reactors which have been allowed to dry out (and over heat) before being recovered with water. In a gap release the activity in the fuel/cladding gap has been released while in the core melt release the core was melted before being recovered by water.
much of the xenon
and iodine
was released while much less of the zirconium
was released. The fact that only the more volatile fission products are released with ease will greatly retard the release of radioactivity in the event of an accident which causes serious damage to the core. Using two sources of data it is possible to see that the elements which were in the form of gases, volatile compounds or semi-volatile compounds (such as CsI) were released at Chernobyl while the less volatile elements which form solid solutions with the fuel remained inside the reactor fuel.
According to the OECD NEA report on Chernobyl (ten years on), the following proportions of the core inventory were released. The physical
and chemical forms of the release included gases, aerosols and finely fragmented solid fuel. According to some research the ruthenium
is very mobile when the nuclear fuel is heated with air.
Some work has been done on TRISO fuel under similar conditions.
The releases of fission products and uranium from uranium dioxide (from spent BWR fuel, burnup
was 65 GWd t−1) which was heated in a Knudsen
cell has been repeated. Fuel was heated in the Knudsen cell both with and without preoxidation in oxygen
at c 650 K. It was found even for the noble gas
es that a high temperature was required to liberate them from the uranium oxide solid. For unoxidized fuel 2300 K was required to release 10% of the uranium while oxidized fuel only requires 1700 K to release 10% of the uranium.
According to the report on Chernobyl used in the above table 3.5% of the following isotopes in the core were released 239Np, 238Pu, 239Pu, 240Pu, 241Pu and 242Cm.
can react violently at 1200 °C, at the same temperature the zircaloy
cladding can react with uranium dioxide to form zirconium oxide and a uranium/zirconium alloy
melt.
some results of the PIE on PHEBUS (FPT2) fuel are reported in section 3.6.
, and was essentially a scale-model of a commercial PWR
. ('Power/volume scaling' was used between the LOFT model, with a 50MWth core, and a commercial plant of 3000MWth).
The original intention (1963–1975) was to study only one or two major (large break) LOCA, since these had been the main concern of US 'rule-making' hearings in the late 1960s and early 1970s. These rules had focussed around a rather stylised large-break accident, and a set of criteria (e.g. for extent of fuel-clad oxidation) set out in 'Appendix K' of 10CFR50 (Code of Federal Regulations). Following the accident at Three Mile Island, detailed modelling of much smaller LOCA became of equal concern.
38 LOFT tests were eventually performed and their scope was broadened to study a wide spectrum of breach sizes. These tests were used to help validate a series of computer codes (such as RELAP-4, RELAP-5 and TRAC) then being developed to calculate the thermal-hydraulics of LOCA.
or FCI when molten 'corium
' contacted water. Many experiments suggested quite low conversion of thermal to mechanical energy, whereas the theoretical models available appeared to suggest that much higher efficiencies were possible. A NEA
/OECD report was written on the subject in 2000 which states that a steam explosion caused by contact of corium with water has four stages.
and zirconium dioxide
was melted in a crucible
before being added to water. The fragmentation of the fuel which results is reported in the Journal of Nuclear Science and Technology.
at the FZK some work has been done on the effect of thermite
on concrete
, this is a simulation of the effect of the molten core of a reactor breaking through the bottom of the pressure vessel
into the containment building
.
(molten core) will cool and change to a solid with time. It is thought that the solid is weathering with time. The solid can be described as Fuel Containing Mass, it is a mixture of sand
, zirconium
and uranium dioxide
which had been heated at a very high temperature until it has melted. The chemical nature of this FCM has been the subject of some research. The amount of fuel left in this form within the plant has been considered. A silicone
polymer has been used to fix the contamination.
The Chernobyl melt was a silicate
melt which did contain inclusions of Zr
/U
phases, molten steel
and high uranium zirconium silicate. The lava flow consists of more than one type of material—a brown lava and a porous ceramic material have been found.
The uranium to zirconium for different parts of the solid differs a lot, in the brown lava a uranium rich phase with a U:Zr ratio of 19:3 to about 38:10 is found. The uranium poor phase in the brown lava has a U:Zr ratio of about 1:10. It is possible from the examination of the Zr/U phases to know the thermal history of the mixture, it can be shown that before the explosion that in part of the core the temperature was higher than 2000 °C. While in some areas the temperature was over 2400–2600 °C.
films can be deposited by reactive sputtering using an argon
and oxygen
mixture at a low pressure
. This has been used to make a layer of the uranium oxide on a gold
surface which was then studied with AC impedance spectroscopy.
electrochemist
Shoesmith the nanoparticle
s of Mo
-Tc
-Ru
-Pd
have a strong effect on the corrosion of uranium dioxide
fuel. For instance his work suggests that when the hydrogen (H2) concentration is high (due to the anaerobic
corrosion of the steel
waste can) the oxidation of hydrogen at the nanoparticles will exert a protective effect on the uranium dioxide. This effect can be thought of as an example of protection by a sacrificial anode where instead of a metal anode
reacting and dissolving it is the hydrogen gas which is consumed.
Uranium dioxide
Uranium dioxide or uranium oxide , also known as urania or uranous oxide, is an oxide of uranium, and is a black, radioactive, crystalline powder that naturally occurs in the mineral uraninite. It is used in nuclear fuel rods in nuclear reactors. A mixture of uranium and plutonium dioxides is used...
nuclear fuel
Nuclear fuel
Nuclear fuel is a material that can be 'consumed' by fission or fusion to derive nuclear energy. Nuclear fuels are the most dense sources of energy available...
behaves during both normal nuclear reactor
Nuclear reactor
A nuclear reactor is a device to initiate and control a sustained nuclear chain reaction. Most commonly they are used for generating electricity and for the propulsion of ships. Usually heat from nuclear fission is passed to a working fluid , which runs through turbines that power either ship's...
operation and under reactor accident conditions such as overheating. Work in this area is often very expensive to conduct, and so has often been performed on a collaborative basis between groups of countries, usually under the aegis of the CSNI.

Cladding
Both the fuel can swell and the cladding which covers the fuel to form a fuel pin can be deformed. It is normal to fill the gap between the fuel and the cladding with heliumHelium
Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
gas to permit better thermal contact between the fuel and the cladding. During use the amount of gas inside the fuel pin can increase because of the formation of noble gas
Noble gas
The noble gases are a group of chemical elements with very similar properties: under standard conditions, they are all odorless, colorless, monatomic gases, with very low chemical reactivity...
es (krypton
Krypton
Krypton is a chemical element with the symbol Kr and atomic number 36. It is a member of Group 18 and Period 4 elements. A colorless, odorless, tasteless noble gas, krypton occurs in trace amounts in the atmosphere, is isolated by fractionally distilling liquified air, and is often used with other...
and xenon
Xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. The element name is pronounced or . A colorless, heavy, odorless noble gas, xenon occurs in the Earth's atmosphere in trace amounts...
) by the fission process. If a Loss-of-coolant accident (LOCA) (e.g. Three Mile Island
Three Mile Island accident
The Three Mile Island accident was a core meltdown in Unit 2 of the Three Mile Island Nuclear Generating Station in Dauphin County, Pennsylvania near Harrisburg, United States in 1979....
) or a Reactivity Initiated Accident (RIA) (e.g. Chernobyl
Chernobyl disaster
The Chernobyl disaster was a nuclear accident that occurred on 26 April 1986 at the Chernobyl Nuclear Power Plant in Ukraine , which was under the direct jurisdiction of the central authorities in Moscow...
or SL-1
SL-1
The SL-1, or Stationary Low-Power Reactor Number One, was a United States Army experimental nuclear power reactor which underwent a steam explosion and meltdown on January 3, 1961, killing its three operators. The direct cause was the improper withdrawal of the central control rod, responsible for...
) occurs then the temperature of this gas can increase. As the fuel pin is sealed the pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
of the gas will increase (PV = nRT) and it is possible to deform and burst the cladding. It has been noticed that both corrosion
Corrosion
Corrosion is the disintegration of an engineered material into its constituent atoms due to chemical reactions with its surroundings. In the most common use of the word, this means electrochemical oxidation of metals in reaction with an oxidant such as oxygen...
and irradiation
Irradiation
Irradiation is the process by which an object is exposed to radiation. The exposure can originate from various sources, including natural sources. Most frequently the term refers to ionizing radiation, and to a level of radiation that will serve a specific purpose, rather than radiation exposure to...
can alter the properties of the zirconium alloy
Zircaloy
Zirconium alloys are solid solutions of zirconium or other metals, a common subgroup having the trade mark Zircaloy. Zirconium has very low absorption cross-section of thermal neutrons, high hardness, ductility and corrosion resistance...
commonly used as cladding, making it brittle
Brittle
A material is brittle if, when subjected to stress, it breaks without significant deformation . Brittle materials absorb relatively little energy prior to fracture, even those of high strength. Breaking is often accompanied by a snapping sound. Brittle materials include most ceramics and glasses ...
. As a result the experiments using unirradated zirconium alloy tubes can be misleading.
According to one paper the following difference between the cladding failure mode of unused and used fuel was seen.
Unirradiated fuel rods were pressurized before being placed in a special reactor at the Japanese Nuclear Safety Research Reactor
Nuclear Safety Research Reactor
The Nuclear Safety Research Reactor is a TRIGA design nuclear Research reactor operated by the Japan Atomic Energy Agency.*First criticality: June 1975...
(NSRR) where they were subjected to a simulated RIA transient. These rods failed after ballooning late in the transient when the cladding temperature was high. The failure of the cladding in these tests was ductile, and it was a burst opening.
The used fuel (61 GW days/tonne
Tonne
The tonne, known as the metric ton in the US , often put pleonastically as "metric tonne" to avoid confusion with ton, is a metric system unit of mass equal to 1000 kilograms. The tonne is not an International System of Units unit, but is accepted for use with the SI...
of uranium) failed early in the transient with a brittle fracture which was a longitudinal crack.
If was found that hydride
Hydride
In chemistry, a hydride is the anion of hydrogen, H−, or, more commonly, a compound in which one or more hydrogen centres have nucleophilic, reducing, or basic properties. In compounds that are regarded as hydrides, hydrogen is bonded to a more electropositive element or group...
d zirconium tube is weaker and the bursting pressure is lower.
The common failure process of fuel in the water-cooled reactors is a transition to film boiling and subsequent ignition of zirconium cladding in the steam. The effects of the intense hot hydrogen reaction product flow on the fuel pellets and on the bundle's wall well represented on the sidebar picture.
Fuel
The nuclear fuelNuclear fuel
Nuclear fuel is a material that can be 'consumed' by fission or fusion to derive nuclear energy. Nuclear fuels are the most dense sources of energy available...
can swell during use, this is because of effects such as bubble formation in the fuel and the damage which occurs to the lattice of the solid. The swelling of the fuel can impose mechanical stresses upon the cladding which covers the fuel. A document on the subject of the swelling of the fuel can be downloaded from the NASA
NASA
The National Aeronautics and Space Administration is the agency of the United States government that is responsible for the nation's civilian space program and for aeronautics and aerospace research...
web site.
Fission gas release
As the fuel is degraded or heated the more volatile fission products which are trapped within the uranium dioxideUranium dioxide
Uranium dioxide or uranium oxide , also known as urania or uranous oxide, is an oxide of uranium, and is a black, radioactive, crystalline powder that naturally occurs in the mineral uraninite. It is used in nuclear fuel rods in nuclear reactors. A mixture of uranium and plutonium dioxides is used...
may become free. For example see.
A report on the release of 85Kr, 106Ru and 137Cs from uranium when air is present has been written. It was found that uranium dioxide was converted to U3O8 between about 300 and 500 °C in air. They report that this process requires some time to start, after the induction time the sample gains mass. The authors report that a layer of U3O7 was present on the uranium dioxide surface during this induction time. They report that 3 to 8% of the krypton
Krypton
Krypton is a chemical element with the symbol Kr and atomic number 36. It is a member of Group 18 and Period 4 elements. A colorless, odorless, tasteless noble gas, krypton occurs in trace amounts in the atmosphere, is isolated by fractionally distilling liquified air, and is often used with other...
-85 was released, and that much less of the ruthenium
Ruthenium
Ruthenium is a chemical element with symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most chemicals. The Russian scientist Karl Ernst Claus discovered the element...
(0.5%) and caesium
Caesium
Caesium or cesium is the chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-gold alkali metal with a melting point of 28 °C , which makes it one of only five elemental metals that are liquid at room temperature...
(2.6 x 10−3%) occurred during the oxidation of the uranium dioxide.
Heat transfer between the cladding and the water
In a water-cooled power reactor (or in a water-filled used fuel store cooling pondCooling pond
A cooling pond is a man-made body of water primarily formed for the purpose of supplying cooling water to a nearby power plant or industrial facility such as a petroleum refinery, pulp and paper mill, chemical plant, steel mill or smelter...
), if a power surge occurs as a result of a reactivity initiated accident, an understanding of the transfer of heat from the surface of the cladding to the water is very useful. In a French study, metal pipe immersed in water (both under typical PWR
Pressurized water reactor
Pressurized water reactors constitute a large majority of all western nuclear power plants and are one of three types of light water reactor , the other types being boiling water reactors and supercritical water reactors...
and pond conditions), was electrically heated to simulate the generation of heat within a fuel pin by nuclear processes. The temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
of the pipe was monitored by thermocouple
Thermocouple
A thermocouple is a device consisting of two different conductors that produce a voltage proportional to a temperature difference between either end of the pair of conductors. Thermocouples are a widely used type of temperature sensor for measurement and control and can also be used to convert a...
s and for the tests conducted under PWR
Pressurized water reactor
Pressurized water reactors constitute a large majority of all western nuclear power plants and are one of three types of light water reactor , the other types being boiling water reactors and supercritical water reactors...
conditions the water entering the larger pipe (14.2 mm diameter) holding the test metal pipe (9.5 mm outside diameter and 600 mm long) was at 280 oC and 15 MPa. The water was flowing past the inner pipe at circa 4 ms−1 and the cladding was subjected to heating at 2200 to 4900 °C s−1 to simulate an RIA. It was found that as the temperature of the cladding increased the rate of heat transfer from the surface of the cladding increased at first as the water boiled at nucleation
Nucleation
Nucleation is the extremely localized budding of a distinct thermodynamic phase. Some examples of phases that may form by way of nucleation in liquids are gaseous bubbles, crystals or glassy regions. Creation of liquid droplets in saturated vapor is also characterized by nucleation...
sites. When the heat flux is greater than the critical heat flux
Critical heat flux
Critical heat flux describes the thermal limit of a phenomenon where a phase change occurs during heating , which suddenly decreases the efficiency of heat transfer, thus causing localised overheating of the heating surface.The Critical heat flux for ignition is the lowest thermal load per unit...
a boiling crisis occurs. This occurs as the temperature of the fuel cladding surface increases so that the surface of the metal was too hot (surface dries out) for nucleation boiling. When the surface dries out the rate of heat transfer
Heat transfer
Heat transfer is a discipline of thermal engineering that concerns the exchange of thermal energy from one physical system to another. Heat transfer is classified into various mechanisms, such as heat conduction, convection, thermal radiation, and phase-change transfer...
decreases, after a further increase in the temperature of the metal surface the boiling resumes but it is now film boiling.
Corrosion on the inside of the cladding
ZirconiumZirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name of zirconium is taken from the mineral zircon. Its atomic mass is 91.224. It is a lustrous, grey-white, strong transition metal that resembles titanium...
alloys can undergo stress corrosion cracking
Stress corrosion cracking
Stress corrosion cracking is the unexpected sudden failure of normally ductile metals subjected to a tensile stress in a corrosive environment, especially at elevated temperature in the case of metals. SCC is highly chemically specific in that certain alloys are likely to undergo SCC only when...
when exposed to iodine, the iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
is formed as a fission product
Fission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus fissions. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons and a large release of energy in the form of heat , gamma rays and neutrinos. The...
which depending on the nature of the fuel can escape from the pellet. It has been shown that iodine causes the rate of cracking in pressurised zircaloy
Zircaloy
Zirconium alloys are solid solutions of zirconium or other metals, a common subgroup having the trade mark Zircaloy. Zirconium has very low absorption cross-section of thermal neutrons, high hardness, ductility and corrosion resistance...
-4 tubing to increase.
Graphite moderated reactors
In the cases of carbon dioxideCarbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
cooled graphite
Graphite
The mineral graphite is one of the allotropes of carbon. It was named by Abraham Gottlob Werner in 1789 from the Ancient Greek γράφω , "to draw/write", for its use in pencils, where it is commonly called lead . Unlike diamond , graphite is an electrical conductor, a semimetal...
moderated reactors such as magnox
Magnox
Magnox is a now obsolete type of nuclear power reactor which was designed and is still in use in the United Kingdom, and was exported to other countries, both as a power plant, and, when operated accordingly, as a producer of plutonium for nuclear weapons...
and AGR
Advanced gas-cooled reactor
An advanced gas-cooled reactor is a type of nuclear reactor. These are the second generation of British gas-cooled reactors, using graphite as the neutron moderator and carbon dioxide as coolant...
power reactors an important corrosion
Corrosion
Corrosion is the disintegration of an engineered material into its constituent atoms due to chemical reactions with its surroundings. In the most common use of the word, this means electrochemical oxidation of metals in reaction with an oxidant such as oxygen...
reaction is the reaction of a molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
of carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
with graphite (carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
) to form two molecules of carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
. This is one of the processes which limits the working life of this type of reactor.
Corrosion
In an water-cooled reactor the action of radiationRadiation
In physics, radiation is a process in which energetic particles or energetic waves travel through a medium or space. There are two distinct types of radiation; ionizing and non-ionizing...
on the water (radiolysis
Radiolysis
Radiolysis is the dissociation of molecules by nuclear radiation. It is the cleavage of one or several chemical bonds resulting from exposure to high-energy flux...
) forms hydrogen peroxide
Hydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
and oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
. These can cause stress corrosion cracking
Stress corrosion cracking
Stress corrosion cracking is the unexpected sudden failure of normally ductile metals subjected to a tensile stress in a corrosive environment, especially at elevated temperature in the case of metals. SCC is highly chemically specific in that certain alloys are likely to undergo SCC only when...
of metal parts which include fuel
Fuel
Fuel is any material that stores energy that can later be extracted to perform mechanical work in a controlled manner. Most fuels used by humans undergo combustion, a redox reaction in which a combustible substance releases energy after it ignites and reacts with the oxygen in the air...
cladding and other pipework. To mitigate this hydrazine
Hydrazine
Hydrazine is an inorganic compound with the formula N2H4. It is a colourless flammable liquid with an ammonia-like odor. Hydrazine is highly toxic and dangerously unstable unless handled in solution. Approximately 260,000 tons are manufactured annually...
and hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
are injected into a BWR or PWR
Pressurized water reactor
Pressurized water reactors constitute a large majority of all western nuclear power plants and are one of three types of light water reactor , the other types being boiling water reactors and supercritical water reactors...
primary cooling circuit as corrosion inhibitor
Corrosion inhibitor
A corrosion inhibitor is a chemical compound that, when added to a liquid or gas, decreases the corrosion rate of a material, typically a metal or an alloy. The effectiveness of a corrosion inhibitor depends on fluid composition, quantity of water, and flow regime...
s to adjust the redox
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
properties of the system. A review of recent developments on this topic has been published.
Thermal stresses upon quenching
In a loss-of-coolant accident (LOCA) it is thought that the surface of the cladding could reach a temperature between 800 and 1400 K, and the cladding will be exposed to steamSteam
Steam is the technical term for water vapor, the gaseous phase of water, which is formed when water boils. In common language it is often used to refer to the visible mist of water droplets formed as this water vapor condenses in the presence of cooler air...
for some time before water is reintroduced into the reactor to cool the fuel. During this time when the hot cladding is exposed to steam some oxidation of the zirconium
Zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name of zirconium is taken from the mineral zircon. Its atomic mass is 91.224. It is a lustrous, grey-white, strong transition metal that resembles titanium...
will occur to form a zirconium oxide which is more zirconium rich than zirconia. This Zr(O) phase is the α-phase, further oxidation forms zirconia. The longer the cladding is exposed to steam the less ductile it will be. One measure of the ductility is to compress a ring along a diameter (at a constant rate of displacement, in this case 2 mm min−1) until the first crack occurs, then the ring will start to fail. The elongation which occurs between when the maximum force is applied and when the mechanical load is declined to 80% of the load required to induce the first crack is the L0.8 value in mm. The more ductile a sample is the greater this L0.8 value will be.
In one experiment the zirconium is heated in steam to 1473 K, the sample is slowly cooled in steam to 1173 K before being quenched in water. As the heating time at 1473 K is increased the zirconium becomes more brittle and the L0.8 value declines.
Aging of steels
IrradiationIrradiation
Irradiation is the process by which an object is exposed to radiation. The exposure can originate from various sources, including natural sources. Most frequently the term refers to ionizing radiation, and to a level of radiation that will serve a specific purpose, rather than radiation exposure to...
causes the properties of steels to become poorer, for instance SS316
Stainless steel
In metallurgy, stainless steel, also known as inox steel or inox from French "inoxydable", is defined as a steel alloy with a minimum of 10.5 or 11% chromium content by mass....
becomes less ductile and less tough
Toughness
In materials science and metallurgy, toughness is the ability of a material to absorb energy and plastically deform without fracturing; Material toughness is defined as the amount of energy per volume that a material can absorb before rupturing...
. Also creep
Creep (deformation)
In materials science, creep is the tendency of a solid material to slowly move or deform permanently under the influence of stresses. It occurs as a result of long term exposure to high levels of stress that are below the yield strength of the material....
and stress corrosion cracking
Stress corrosion cracking
Stress corrosion cracking is the unexpected sudden failure of normally ductile metals subjected to a tensile stress in a corrosive environment, especially at elevated temperature in the case of metals. SCC is highly chemically specific in that certain alloys are likely to undergo SCC only when...
become worse. Papers on this effect continue to be published.
Cracking and overheating of the fuel
This is due to the fact that as the fuel expands on heatingThermal expansion
Thermal expansion is the tendency of matter to change in volume in response to a change in temperature.When a substance is heated, its particles begin moving more and thus usually maintain a greater average separation. Materials which contract with increasing temperature are rare; this effect is...
, the core of the pellet expands more than the rim. Because of the thermal stress thus formed the fuel cracks, the cracks tend to go from the center to the edge in a star shaped pattern. A PhD thesis on the subject has been published by a student at the Royal Institute of Technology
Royal Institute of Technology
The Royal Institute of Technology is a university in Stockholm, Sweden. KTH was founded in 1827 as Sweden's first polytechnic and is one of Scandinavia's largest institutions of higher education in technology. KTH accounts for one-third of Sweden’s technical research and engineering education...
in Stockholm
Stockholm
Stockholm is the capital and the largest city of Sweden and constitutes the most populated urban area in Scandinavia. Stockholm is the most populous city in Sweden, with a population of 851,155 in the municipality , 1.37 million in the urban area , and around 2.1 million in the metropolitan area...
(Sweden
Sweden
Sweden , officially the Kingdom of Sweden , is a Nordic country on the Scandinavian Peninsula in Northern Europe. Sweden borders with Norway and Finland and is connected to Denmark by a bridge-tunnel across the Öresund....
).
The cracking of the fuel has an effect on the release of radioactivity from fuel both under accident conditions and also when the spent fuel is used as the final disposal form. The cracking increases the surface area of the fuel which increases the rate at which fission products can leave the fuel.
The temperature of the fuel varies as a function of the distance from the center to the rim. At distance x from the center the temperature (Tx) is described by the equation
Equation
An equation is a mathematical statement that asserts the equality of two expressions. In modern notation, this is written by placing the expressions on either side of an equals sign , for examplex + 3 = 5\,asserts that x+3 is equal to 5...
where ρ is the power density (W m−3) and Kf is the thermal conductivity
Thermal conductivity
In physics, thermal conductivity, k, is the property of a material's ability to conduct heat. It appears primarily in Fourier's Law for heat conduction....
.
Tx = TRim + ρ (rpellet² – x²) (4 Kf)−1
To explain this a for a series of fuel pellets being used with a rim temperature of 200 °C (typical for a BWR) with different diameters and power densities of 250 Wm−3 have been modeled using the above equation. These fuel pellets are rather large; it is normal to use oxide pellets which are about 10 mm in diameter.



Power density
Power density is the amount of power per unit volume....
. It is likely that the maths used for these calculations would be used to explain how electrical fuses
Fuse (electrical)
In electronics and electrical engineering, a fuse is a type of low resistance resistor that acts as a sacrificial device to provide overcurrent protection, of either the load or source circuit...
function and also it could be used to predict the centerline temperature in any system where heat is released throughout a cylinder shaped object.


Loss of volatile fission products from pellets
The heating of pellets can result in some of the fission products being lost from the core of the pellet. If the xenon can rapidly leave the pellet then the amount of 134Cs and 137Cs which is present in the gap between the cladding and the fuel will increase. As a result if the zircaloyZircaloy
Zirconium alloys are solid solutions of zirconium or other metals, a common subgroup having the trade mark Zircaloy. Zirconium has very low absorption cross-section of thermal neutrons, high hardness, ductility and corrosion resistance...
tubes holding the pellet are broken then a greater release of radioactive caesium from the fuel will occur. It is important to understand that the 134Cs and 137Cs are formed in different ways, and hence as a result the two caesium isotopes can be found at different parts of a fuel pin.
It is clear that the volatile iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
and xenon
Xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. The element name is pronounced or . A colorless, heavy, odorless noble gas, xenon occurs in the Earth's atmosphere in trace amounts...
isotopes have minutes in which they can diffuse out of the pellet and into the gap between the fuel and the cladding. Here the xenon can decay to the long lived caesium isotope.
Genesis of 137Cs
| Element | Isotope | decay mode | half life | direct fission yield |
|---|---|---|---|---|
| Sn Tin Tin is a chemical element with the symbol Sn and atomic number 50. It is a main group metal in group 14 of the periodic table. Tin shows chemical similarity to both neighboring group 14 elements, germanium and lead and has two possible oxidation states, +2 and the slightly more stable +4... |
137 | β | very short (<1 s) | 0.00% |
| Sb Antimony Antimony is a toxic chemical element with the symbol Sb and an atomic number of 51. A lustrous grey metalloid, it is found in nature mainly as the sulfide mineral stibnite... |
137 | β | very short (<1 s) | 0.03% |
| Te | 137 | β | 2.5 seconds | 0.19% |
| I Iodine Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor.... |
137 | β | 24.5 seconds | 1.40% |
| Xe Xenon Xenon is a chemical element with the symbol Xe and atomic number 54. The element name is pronounced or . A colorless, heavy, odorless noble gas, xenon occurs in the Earth's atmosphere in trace amounts... |
137 | β | 3.8 minutes | 1.44% |
| Cs Caesium Caesium or cesium is the chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-gold alkali metal with a melting point of 28 °C , which makes it one of only five elemental metals that are liquid at room temperature... |
137 | β | 30 years | 0.08% |
These fission yields were calculated for 235U assuming thermal neutrons (0.0253 eV) using data from the chart of the nuclides.
Genesis of 134Cs
In the case of 134Cs the precursor to this isotope is stable 133Cs which is formed by the decay of much longer lived xenon and iodine isotopes. No 134Cs is formed without neutron activationNeutron activation
Neutron activation is the process in which neutron radiation induces radioactivity in materials, and occurs when atomic nuclei capture free neutrons, becoming heavier and entering excited states. The excited nucleus often decays immediately by emitting particles such as neutrons, protons, or alpha...
as 134Xe is a stable isotope. As a result of this different mode of formation the physical location of 134Cs can differ from that of 137Cs.
| Element | Isotope | decay mode | half life | direct fission yield |
|---|---|---|---|---|
| In Indium Indium is a chemical element with the symbol In and atomic number 49. This rare, very soft, malleable and easily fusible post-transition metal is chemically similar to gallium and thallium, and shows the intermediate properties between these two... |
133 | β | 0.18 seconds | 0.00% |
| Sn Tin Tin is a chemical element with the symbol Sn and atomic number 50. It is a main group metal in group 14 of the periodic table. Tin shows chemical similarity to both neighboring group 14 elements, germanium and lead and has two possible oxidation states, +2 and the slightly more stable +4... |
133 | β | 1.45 seconds | 0.07% |
| Sb Antimony Antimony is a toxic chemical element with the symbol Sb and an atomic number of 51. A lustrous grey metalloid, it is found in nature mainly as the sulfide mineral stibnite... |
133 | β | 2.5 minutes | 1.11% |
| Te | 133m | β (82.5%) | 55.4 minutes | 0.49% |
| Te | 133 | β | 12.5 minutes | 0.15% |
| I Iodine Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor.... |
133 | β | 20.8 hours | 1.22% |
| Xe Xenon Xenon is a chemical element with the symbol Xe and atomic number 54. The element name is pronounced or . A colorless, heavy, odorless noble gas, xenon occurs in the Earth's atmosphere in trace amounts... |
133 | β | 5.2 days | 0.00% |
| Cs Caesium Caesium or cesium is the chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-gold alkali metal with a melting point of 28 °C , which makes it one of only five elemental metals that are liquid at room temperature... |
133 | – | stable (undergoes neutron activation in the core) | 0.00% |
| Cs Caesium Caesium or cesium is the chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-gold alkali metal with a melting point of 28 °C , which makes it one of only five elemental metals that are liquid at room temperature... |
134 | β | 2.1 years | 6.4 x 10−6% |
These fission yields were calculated for 235U assuming thermal neutrons (0.0253 eV) using data from the chart of the nuclides.
An example of a recent PIE study
In a recent study, used 20% enriched uranium dispersed in a range of different matrices was examined to determine the physical locations of different isotopes and chemical elements.- A solid solutionSolid solutionA solid solution is a solid-state solution of one or more solutes in a solvent. Such a mixture is considered a solution rather than a compound when the crystal structure of the solvent remains unchanged by addition of the solutes, and when the mixture remains in a single homogeneous phase...
of uraniaUranium dioxideUranium dioxide or uranium oxide , also known as urania or uranous oxide, is an oxide of uranium, and is a black, radioactive, crystalline powder that naturally occurs in the mineral uraninite. It is used in nuclear fuel rods in nuclear reactors. A mixture of uranium and plutonium dioxides is used...
in yttria-stabilized zirconiaYttria-stabilized zirconiaYttria-stabilized zirconia is a zirconium-oxide based ceramic, in which the particular crystal structure of zirconium oxide is made stable at room temperature by an addition of yttrium oxide...
(YSZ) {Y:Zr atom ratio of 1:4}). - UraniaUranium dioxideUranium dioxide or uranium oxide , also known as urania or uranous oxide, is an oxide of uranium, and is a black, radioactive, crystalline powder that naturally occurs in the mineral uraninite. It is used in nuclear fuel rods in nuclear reactors. A mixture of uranium and plutonium dioxides is used...
particles in an inert matrix formed by a mixture of YSZ and spinelSpinelSpinel is the magnesium aluminium member of the larger spinel group of minerals. It has the formula MgAl2O4. Balas ruby is an old name for a rose-tinted variety.-Spinel group:...
(MgAl2O4). - Urania particles dispersed in the inert matrix formed by a mixture of YSZ and alumina.
The fuels varied in their ability to retain the fission xenon
Xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. The element name is pronounced or . A colorless, heavy, odorless noble gas, xenon occurs in the Earth's atmosphere in trace amounts...
; the first of the three fuels retained 97% of the 133Xe, the second retained 94% while the last fuel only retained 76% of this xenon isotope. The 133Xe is a long-lived radioactive isotope which can diffuse slowly out of the pellet before being neutron activated
Neutron activation
Neutron activation is the process in which neutron radiation induces radioactivity in materials, and occurs when atomic nuclei capture free neutrons, becoming heavier and entering excited states. The excited nucleus often decays immediately by emitting particles such as neutrons, protons, or alpha...
to form 134Cs. The more short-lived 137Xe was less able to leach out of the pellets; 99%, 98% and 95% of the 137Xe was retained within the pellets. It was also found that the 137Cs
Caesium-137
Caesium-137 is a radioactive isotope of caesium which is formed as a fission product by nuclear fission.It has a half-life of about 30.17 years, and decays by beta emission to a metastable nuclear isomer of barium-137: barium-137m . Caesium-137 is a radioactive isotope of caesium which is formed...
concentration in the core of the pellet was much lower than the concentration in the rim of the pellet, while the less volatile 106Ru was spread more evenly throughout the pellets.
The following fuel is particles of solid solution
Solid solution
A solid solution is a solid-state solution of one or more solutes in a solvent. Such a mixture is considered a solution rather than a compound when the crystal structure of the solvent remains unchanged by addition of the solutes, and when the mixture remains in a single homogeneous phase...
of urania in yttria-stabilized zirconia
Yttria-stabilized zirconia
Yttria-stabilized zirconia is a zirconium-oxide based ceramic, in which the particular crystal structure of zirconium oxide is made stable at room temperature by an addition of yttrium oxide...
dispersed in alumina which had burnt up
Burnup
In nuclear power technology, burnup is a measure of how much energy is extracted from a primary nuclear fuel source...
to 105 GW-days per cubic meter. The scanning electron microscope
Scanning electron microscope
A scanning electron microscope is a type of electron microscope that images a sample by scanning it with a high-energy beam of electrons in a raster scan pattern...
(SEM) is of the interface between the alumina and a fuel particle. It can be seen that the fission products are well confined to within the fuel, little of the fission products have entered the alumina matrix. The neodymium
Neodymium
Neodymium is a chemical element with the symbol Nd and atomic number 60. It is a soft silvery metal that tarnishes in air. Neodymium was discovered in 1885 by the Austrian chemist Carl Auer von Welsbach. It is present in significant quantities in the ore minerals monazite and bastnäsite...
is spread throughout the fuel in a uniform manner, while the caesium
Caesium
Caesium or cesium is the chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-gold alkali metal with a melting point of 28 °C , which makes it one of only five elemental metals that are liquid at room temperature...
is almost homogenously spread out throughout the fuel. The caesium concentration is slightly higher at two points where xenon bubbles are present. Much of the xenon is present in bubbles, while almost all of the ruthenium
Ruthenium
Ruthenium is a chemical element with symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most chemicals. The Russian scientist Karl Ernst Claus discovered the element...
is present in the form of nanoparticle
Nanoparticle
In nanotechnology, a particle is defined as a small object that behaves as a whole unit in terms of its transport and properties. Particles are further classified according to size : in terms of diameter, coarse particles cover a range between 10,000 and 2,500 nanometers. Fine particles are sized...
s. The ruthenium nanoparticles are not always colocated with the xenon bubbles.
Release of fission products into coolant water in a Three Mile Island type accident
At Three Mile IslandThree Mile Island accident
The Three Mile Island accident was a core meltdown in Unit 2 of the Three Mile Island Nuclear Generating Station in Dauphin County, Pennsylvania near Harrisburg, United States in 1979....
a recently SCRAM
Scram
A scram or SCRAM is an emergency shutdown of a nuclear reactor – though the term has been extended to cover shutdowns of other complex operations, such as server farms and even large model railroads...
ed core was starved of cooling water, as a result of the decay heat
Decay heat
Decay heat is the heat released as a result of radioactive decay. This is when the radiation interacts with materials: the energy of the alpha, beta or gamma radiation is converted into the thermal movement of atoms.-Natural occurrence:...
the core dried out and the fuel was damaged. Attempts were made to recool the core using water. According to the International Atomic Energy Agency
International Atomic Energy Agency
The International Atomic Energy Agency is an international organization that seeks to promote the peaceful use of nuclear energy, and to inhibit its use for any military purpose, including nuclear weapons. The IAEA was established as an autonomous organization on 29 July 1957...
for a 3,000 MW(t) PWR
Pressurized water reactor
Pressurized water reactors constitute a large majority of all western nuclear power plants and are one of three types of light water reactor , the other types being boiling water reactors and supercritical water reactors...
the normal coolant radioactivity levels are shown below in the table, and the coolant activities for reactors which have been allowed to dry out (and over heat) before being recovered with water. In a gap release the activity in the fuel/cladding gap has been released while in the core melt release the core was melted before being recovered by water.
| Isotope | Normal | >20% Gap release | >10% Core melt |
|---|---|---|---|
| 131I Iodine Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor.... |
2 | 200000 | 700000 |
| 134Cs Caesium Caesium or cesium is the chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-gold alkali metal with a melting point of 28 °C , which makes it one of only five elemental metals that are liquid at room temperature... |
0.3 | 10000 | 60000 |
| 137Cs Caesium Caesium or cesium is the chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-gold alkali metal with a melting point of 28 °C , which makes it one of only five elemental metals that are liquid at room temperature... |
0.3 | 6000 | 30000 |
| 140Ba Barium Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in Group 2, a soft silvery metallic alkaline earth metal. Barium is never found in nature in its pure form due to its reactivity with air. Its oxide is historically known as baryta but it reacts with... |
0.5 | – | 100000 |
Chernobyl release
The release of radioactivity from the used fuel is greatly controlled by the volatility of the elements. At ChernobylChernobyl disaster
The Chernobyl disaster was a nuclear accident that occurred on 26 April 1986 at the Chernobyl Nuclear Power Plant in Ukraine , which was under the direct jurisdiction of the central authorities in Moscow...
much of the xenon
Xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. The element name is pronounced or . A colorless, heavy, odorless noble gas, xenon occurs in the Earth's atmosphere in trace amounts...
and iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
was released while much less of the zirconium
Zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name of zirconium is taken from the mineral zircon. Its atomic mass is 91.224. It is a lustrous, grey-white, strong transition metal that resembles titanium...
was released. The fact that only the more volatile fission products are released with ease will greatly retard the release of radioactivity in the event of an accident which causes serious damage to the core. Using two sources of data it is possible to see that the elements which were in the form of gases, volatile compounds or semi-volatile compounds (such as CsI) were released at Chernobyl while the less volatile elements which form solid solutions with the fuel remained inside the reactor fuel.
According to the OECD NEA report on Chernobyl (ten years on), the following proportions of the core inventory were released. The physical
Physics
Physics is a natural science that involves the study of matter and its motion through spacetime, along with related concepts such as energy and force. More broadly, it is the general analysis of nature, conducted in order to understand how the universe behaves.Physics is one of the oldest academic...
and chemical forms of the release included gases, aerosols and finely fragmented solid fuel. According to some research the ruthenium
Ruthenium
Ruthenium is a chemical element with symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most chemicals. The Russian scientist Karl Ernst Claus discovered the element...
is very mobile when the nuclear fuel is heated with air.
Some work has been done on TRISO fuel under similar conditions.
Table of chemical data
| Element | Gas | Metal | Oxide | Solid solution | Radioisotopes | Release at Chernobyl | T required for 10% release from UO2 | T required for 10% release from U3O8 |
|---|---|---|---|---|---|---|---|---|
| Br | Yes | – | – | – | – | – | – | – |
| Kr | Yes | – | – | – | 85Kr | 100% | – | – |
| Rb | Yes | – | Yes | – | – | – | – | – |
| Sr | – | – | Yes | Yes | 89Sr and 90Sr | 4–6% | 1950 K | – |
| Y | – | – | – | Yes | – | 3.5% | – | – |
| Zr | – | – | Yes | Yes | 95Zr | 3.5% | 2600 K | – |
| Nb | – | – | Yes | – | – | – | – | – |
| Mo | – | Yes | Yes | – | 99Mo | >3.5% | – | 1200 K |
| Tc | – | Yes | – | – | – | – | – | 1300 K |
| Ru | – | Yes | – | – | 103Ru and 106Ru | >3.5% | – | – |
| Rh | – | Yes | – | – | – | – | – | – |
| Pd | – | Yes | – | – | – | – | – | – |
| Ag | – | Yes | – | – | – | – | – | – |
| Cd | – | Yes | – | – | – | – | – | – |
| In | – | Yes | – | – | – | – | – | – |
| Sn | – | Yes | – | – | – | – | – | – |
| Sb | – | Yes | – | – | – | – | – | – |
| Te | Yes | Yes | Yes | Yes | 132Te | 25–60% | 1400 K | 1200 K |
| I | Yes | – | – | – | 131I | 50–60% | 1300 K | 1100 K |
| Xe | Yes | – | – | – | 133Xe | 100% | 1450 K | – |
| Cs | Yes | – | Yes | – | 134Cs and 137Cs | 20–40% | 1300 K | 1200 to 1300 K |
| Ba | – | – | Yes | Yes | 140Ba | 4–6% | 1850 K | 1300 K |
| La | – | – | – | Yes | – | 3.5% | 2300 K | – |
| Ce | – | – | – | Yes | 141Ce and 144Ce | 3.5% | 2300 K | – |
| Pr | – | – | – | Yes | – | 3.5% | 2300 K | – |
| Nd | – | – | – | Yes | – | 3.5% | 2300 K | – |
| Pm | – | – | – | Yes | – | 3.5% | 2300 K | – |
| Sm | – | – | – | Yes | – | 3.5% | 2300 K | – |
| Eu | – | – | – | Yes | – | 3.5% | 2300 K | – |
The releases of fission products and uranium from uranium dioxide (from spent BWR fuel, burnup
Burnup
In nuclear power technology, burnup is a measure of how much energy is extracted from a primary nuclear fuel source...
was 65 GWd t−1) which was heated in a Knudsen
Martin Knudsen
This article is about the Danish physicist Martin Knudsen. For the Norwegian footballer, see Martin Knudsen .Martin Hans Christian Knudsen was a Danish physicist who taught and conducted research at the Technical University of DenmarkHe is primarily known for his study of molecular gas flow and the...
cell has been repeated. Fuel was heated in the Knudsen cell both with and without preoxidation in oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
at c 650 K. It was found even for the noble gas
Noble gas
The noble gases are a group of chemical elements with very similar properties: under standard conditions, they are all odorless, colorless, monatomic gases, with very low chemical reactivity...
es that a high temperature was required to liberate them from the uranium oxide solid. For unoxidized fuel 2300 K was required to release 10% of the uranium while oxidized fuel only requires 1700 K to release 10% of the uranium.
According to the report on Chernobyl used in the above table 3.5% of the following isotopes in the core were released 239Np, 238Pu, 239Pu, 240Pu, 241Pu and 242Cm.
Degradation of the whole fuel element
Water and zirconiumZirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name of zirconium is taken from the mineral zircon. Its atomic mass is 91.224. It is a lustrous, grey-white, strong transition metal that resembles titanium...
can react violently at 1200 °C, at the same temperature the zircaloy
Zircaloy
Zirconium alloys are solid solutions of zirconium or other metals, a common subgroup having the trade mark Zircaloy. Zirconium has very low absorption cross-section of thermal neutrons, high hardness, ductility and corrosion resistance...
cladding can react with uranium dioxide to form zirconium oxide and a uranium/zirconium alloy
Alloy
An alloy is a mixture or metallic solid solution composed of two or more elements. Complete solid solution alloys give single solid phase microstructure, while partial solutions give two or more phases that may or may not be homogeneous in distribution, depending on thermal history...
melt.
PHEBUS
In France a facility exists in which a fuel melting incident can be made to happen under strictly controlled conditions. In the PHEBUS research program fuels have been allowed to heat up to temperatures in excess of the normal operating temperatures, the fuel in question is in a special channel which is in a toroidal nuclear reactor. The nuclear reactor is used as a driver core to irradate the test fuel. While the reactor is cooled as normal by its own cooling system the test fuel has its own cooling system, which is fitted with filters and equipment to study the release of radioactivity from the damaged fuel. Already the release of radioisotopes from fuel under different conditions has been studied. After the fuel has been used in the experiment it is subject to a detailed examination (PIE), In the 2004 annual report from the ITUItu
Itu is an old and historic municipality in the state of São Paulo in Brazil. The population in 2009 was 157,384 and the area is 641.68 km². The elevation is 583 m. This place name comes from the Tupi language, meaning big waterfall. Itu is linked with the highway numbered the SP-75 and are flowed...
some results of the PIE on PHEBUS (FPT2) fuel are reported in section 3.6.
LOFT
The Loss of Fluid Tests (LOFT) were an early attempt to scope the response of real nuclear fuel to conditions under a loss-of-coolant accident, funded by USNRC. The facility was built at Idaho National LaboratoryIdaho National Laboratory
Idaho National Laboratory is an complex located in the high desert of eastern Idaho, between the town of Arco to the west and the cities of Idaho Falls and Blackfoot to the east. It lies within Butte, Bingham, Bonneville and Jefferson counties...
, and was essentially a scale-model of a commercial PWR
Pressurized water reactor
Pressurized water reactors constitute a large majority of all western nuclear power plants and are one of three types of light water reactor , the other types being boiling water reactors and supercritical water reactors...
. ('Power/volume scaling' was used between the LOFT model, with a 50MWth core, and a commercial plant of 3000MWth).
The original intention (1963–1975) was to study only one or two major (large break) LOCA, since these had been the main concern of US 'rule-making' hearings in the late 1960s and early 1970s. These rules had focussed around a rather stylised large-break accident, and a set of criteria (e.g. for extent of fuel-clad oxidation) set out in 'Appendix K' of 10CFR50 (Code of Federal Regulations). Following the accident at Three Mile Island, detailed modelling of much smaller LOCA became of equal concern.
38 LOFT tests were eventually performed and their scope was broadened to study a wide spectrum of breach sizes. These tests were used to help validate a series of computer codes (such as RELAP-4, RELAP-5 and TRAC) then being developed to calculate the thermal-hydraulics of LOCA.
Water
Extensive work was done from 1970 to 1990 on the possibility of a steam explosionSteam explosion
A steam explosion is a violent boiling or flashing of water into steam, occurring when water is either superheated, rapidly heated by fine hot debris produced within it, or the interaction of molten metals A steam explosion (also called a littoral explosion, or fuel-coolant interaction, FCI) is a...
or FCI when molten 'corium
Corium (nuclear reactor)
Corium, also called fuel containing material or lava-like fuel containing material , is a lava-like molten mixture of portions of nuclear reactor core, formed during a nuclear meltdown, the most severe class of a nuclear reactor accident...
' contacted water. Many experiments suggested quite low conversion of thermal to mechanical energy, whereas the theoretical models available appeared to suggest that much higher efficiencies were possible. A NEA
Nuclear Energy Agency
The Nuclear Energy Agency is an intergovernmental multinational agency that is organized under the Organisation for Economic Co-operation and Development...
/OECD report was written on the subject in 2000 which states that a steam explosion caused by contact of corium with water has four stages.
- Premixing
- As the jet of corium enters the water, it breaks up into droplets. During this stage the thermal contact between the corium and the water is not good because a vapor film surrounds the droplets of corium and this insulates the two from each other. It is possible for this meta-stable state to quench without an explosion or it can trigger in the next step
- Triggering
- A externally or internally generated trigger (such as a pressure wave) causes a collapse of the vapor film between the corium and the water.
- Propagation
- The local increase in pressure due to the increased heating of the water can generate enhanced heat transferHeat transferHeat transfer is a discipline of thermal engineering that concerns the exchange of thermal energy from one physical system to another. Heat transfer is classified into various mechanisms, such as heat conduction, convection, thermal radiation, and phase-change transfer...
(usually due to rapid fragmentation of the hot fluid within the colder more volatile one) and a greater pressure wave, this process can be self-sustained. (The mechanics of this stage would then be similar to those in a classical ZND detonation wave).
- The local increase in pressure due to the increased heating of the water can generate enhanced heat transfer
- Expansion
- This process leads to the whole of the water being suddenly heated to boiling. This causes an increase in pressure which can result in damage to the plant.
Recent work
Some work has been done in Japan where uranium dioxideUranium dioxide
Uranium dioxide or uranium oxide , also known as urania or uranous oxide, is an oxide of uranium, and is a black, radioactive, crystalline powder that naturally occurs in the mineral uraninite. It is used in nuclear fuel rods in nuclear reactors. A mixture of uranium and plutonium dioxides is used...
and zirconium dioxide
Zirconium dioxide
Zirconium dioxide , sometimes known as zirconia , is a white crystalline oxide of zirconium. Its most naturally occurring form, with a monoclinic crystalline structure, is the rare mineral baddeleyite. The high temperature cubic crystalline form is rarely found in nature as mineral tazheranite O2...
was melted in a crucible
Crucible
A crucible is a container used for metal, glass, and pigment production as well as a number of modern laboratory processes, which can withstand temperatures high enough to melt or otherwise alter its contents...
before being added to water. The fragmentation of the fuel which results is reported in the Journal of Nuclear Science and Technology.
Concrete
A review of the subject can be read at and work on the subject continues to this day; in GermanyGermany
Germany , officially the Federal Republic of Germany , is a federal parliamentary republic in Europe. The country consists of 16 states while the capital and largest city is Berlin. Germany covers an area of 357,021 km2 and has a largely temperate seasonal climate...
at the FZK some work has been done on the effect of thermite
Thermite
Thermite is a pyrotechnic composition of a metal powder and a metal oxide that produces an exothermic oxidation-reduction reaction known as a thermite reaction. If aluminium is the reducing agent it is called an aluminothermic reaction...
on concrete
Concrete
Concrete is a composite construction material, composed of cement and other cementitious materials such as fly ash and slag cement, aggregate , water and chemical admixtures.The word concrete comes from the Latin word...
, this is a simulation of the effect of the molten core of a reactor breaking through the bottom of the pressure vessel
Reactor vessel
In a nuclear power plant, the reactor vessel is a pressure vessel containing the Nuclear reactor coolant and reactor core.Not all power reactors have a reactor vessel. Power reactors are generally classified by the type of coolant rather than by the configuration of the reactor vessel used to...
into the containment building
Containment building
A containment building, in its most common usage, is a steel or reinforced concrete structure enclosing a nuclear reactor. It is designed, in any emergency, to contain the escape of radiation to a maximum pressure in the range of 60 to 200 psi...
.
Lava flows from corium
It is possible to see in the photo shown below that the coriumCorium (nuclear reactor)
Corium, also called fuel containing material or lava-like fuel containing material , is a lava-like molten mixture of portions of nuclear reactor core, formed during a nuclear meltdown, the most severe class of a nuclear reactor accident...
(molten core) will cool and change to a solid with time. It is thought that the solid is weathering with time. The solid can be described as Fuel Containing Mass, it is a mixture of sand
Sand
Sand is a naturally occurring granular material composed of finely divided rock and mineral particles.The composition of sand is highly variable, depending on the local rock sources and conditions, but the most common constituent of sand in inland continental settings and non-tropical coastal...
, zirconium
Zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name of zirconium is taken from the mineral zircon. Its atomic mass is 91.224. It is a lustrous, grey-white, strong transition metal that resembles titanium...
and uranium dioxide
Uranium dioxide
Uranium dioxide or uranium oxide , also known as urania or uranous oxide, is an oxide of uranium, and is a black, radioactive, crystalline powder that naturally occurs in the mineral uraninite. It is used in nuclear fuel rods in nuclear reactors. A mixture of uranium and plutonium dioxides is used...
which had been heated at a very high temperature until it has melted. The chemical nature of this FCM has been the subject of some research. The amount of fuel left in this form within the plant has been considered. A silicone
Silicone
Silicones are inert, synthetic compounds with a variety of forms and uses. Typically heat-resistant and rubber-like, they are used in sealants, adhesives, lubricants, medical applications , cookware, and insulation....
polymer has been used to fix the contamination.
The Chernobyl melt was a silicate
Silicate
A silicate is a compound containing a silicon bearing anion. The great majority of silicates are oxides, but hexafluorosilicate and other anions are also included. This article focuses mainly on the Si-O anions. Silicates comprise the majority of the earth's crust, as well as the other...
melt which did contain inclusions of Zr
Zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name of zirconium is taken from the mineral zircon. Its atomic mass is 91.224. It is a lustrous, grey-white, strong transition metal that resembles titanium...
/U
Uranium
Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
phases, molten steel
Steel
Steel is an alloy that consists mostly of iron and has a carbon content between 0.2% and 2.1% by weight, depending on the grade. Carbon is the most common alloying material for iron, but various other alloying elements are used, such as manganese, chromium, vanadium, and tungsten...
and high uranium zirconium silicate. The lava flow consists of more than one type of material—a brown lava and a porous ceramic material have been found.
The uranium to zirconium for different parts of the solid differs a lot, in the brown lava a uranium rich phase with a U:Zr ratio of 19:3 to about 38:10 is found. The uranium poor phase in the brown lava has a U:Zr ratio of about 1:10. It is possible from the examination of the Zr/U phases to know the thermal history of the mixture, it can be shown that before the explosion that in part of the core the temperature was higher than 2000 °C. While in some areas the temperature was over 2400–2600 °C.
Uranium dioxide films
Uranium dioxideUranium dioxide
Uranium dioxide or uranium oxide , also known as urania or uranous oxide, is an oxide of uranium, and is a black, radioactive, crystalline powder that naturally occurs in the mineral uraninite. It is used in nuclear fuel rods in nuclear reactors. A mixture of uranium and plutonium dioxides is used...
films can be deposited by reactive sputtering using an argon
Argon
Argon is a chemical element represented by the symbol Ar. Argon has atomic number 18 and is the third element in group 18 of the periodic table . Argon is the third most common gas in the Earth's atmosphere, at 0.93%, making it more common than carbon dioxide...
and oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
mixture at a low pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
. This has been used to make a layer of the uranium oxide on a gold
Gold
Gold is a chemical element with the symbol Au and an atomic number of 79. Gold is a dense, soft, shiny, malleable and ductile metal. Pure gold has a bright yellow color and luster traditionally considered attractive, which it maintains without oxidizing in air or water. Chemically, gold is a...
surface which was then studied with AC impedance spectroscopy.
Noble metal nanoparticles and hydrogen
According to the work of the corrosionCorrosion
Corrosion is the disintegration of an engineered material into its constituent atoms due to chemical reactions with its surroundings. In the most common use of the word, this means electrochemical oxidation of metals in reaction with an oxidant such as oxygen...
electrochemist
Electrochemistry
Electrochemistry is a branch of chemistry that studies chemical reactions which take place in a solution at the interface of an electron conductor and an ionic conductor , and which involve electron transfer between the electrode and the electrolyte or species in solution.If a chemical reaction is...
Shoesmith the nanoparticle
Nanoparticle
In nanotechnology, a particle is defined as a small object that behaves as a whole unit in terms of its transport and properties. Particles are further classified according to size : in terms of diameter, coarse particles cover a range between 10,000 and 2,500 nanometers. Fine particles are sized...
s of Mo
Molybdenum
Molybdenum , is a Group 6 chemical element with the symbol Mo and atomic number 42. The name is from Neo-Latin Molybdaenum, from Ancient Greek , meaning lead, itself proposed as a loanword from Anatolian Luvian and Lydian languages, since its ores were confused with lead ores...
-Tc
Technetium
Technetium is the chemical element with atomic number 43 and symbol Tc. It is the lowest atomic number element without any stable isotopes; every form of it is radioactive. Nearly all technetium is produced synthetically and only minute amounts are found in nature...
-Ru
Ruthenium
Ruthenium is a chemical element with symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most chemicals. The Russian scientist Karl Ernst Claus discovered the element...
-Pd
Palladium
Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
have a strong effect on the corrosion of uranium dioxide
Uranium dioxide
Uranium dioxide or uranium oxide , also known as urania or uranous oxide, is an oxide of uranium, and is a black, radioactive, crystalline powder that naturally occurs in the mineral uraninite. It is used in nuclear fuel rods in nuclear reactors. A mixture of uranium and plutonium dioxides is used...
fuel. For instance his work suggests that when the hydrogen (H2) concentration is high (due to the anaerobic
Hypoxia (environmental)
Hypoxia, or oxygen depletion, is a phenomenon that occurs in aquatic environments as dissolved oxygen becomes reduced in concentration to a point where it becomes detrimental to aquatic organisms living in the system...
corrosion of the steel
Steel
Steel is an alloy that consists mostly of iron and has a carbon content between 0.2% and 2.1% by weight, depending on the grade. Carbon is the most common alloying material for iron, but various other alloying elements are used, such as manganese, chromium, vanadium, and tungsten...
waste can) the oxidation of hydrogen at the nanoparticles will exert a protective effect on the uranium dioxide. This effect can be thought of as an example of protection by a sacrificial anode where instead of a metal anode
Anode
An anode is an electrode through which electric current flows into a polarized electrical device. Mnemonic: ACID ....
reacting and dissolving it is the hydrogen gas which is consumed.
External links
LOFT tests- INEL News Idaho National Engineering Laboratory, 4 December 1979
- LOFT L2-3 tests completed successfully, Idaho National Engineering Laboratory, June 1979
- Second loss of fluid small break test conducted, Idaho National Engineering Laboratory, February 1980
- http://www.inl.gov/threemileisland/docs/1980-july-loss-of-fluid-test-successfully-completed-organizations-compile-tmi-data.pdf http://www.inl.gov/threemileisland/docs/1980-june-loft-conducts-tmi-type-test.pdf http://www.inl.gov/threemileisland/docs/1982-january-semiscale-tests-reactor-coolant-level-measurement-system.pdf http://www.inl.gov/threemileisland/docs/1983-april-pbf-fuel-damage-test-slated.pdf http://www.inl.gov/threemileisland/docs/1983-september-severe-fuel-damage-test-successful.pdf http://www.inl.gov/threemileisland/docs/1984-january-recap-large-break-loss-of-coolant-accident.pdf http://www.inl.gov/threemileisland/docs/1985-january-1984-recap-including-loss-of-fluid-tests.pdf

