
Plasma polymerization
Encyclopedia
Plasma polymerization uses plasma sources to generate a gas discharge that provides energy to activate or fragment
gaseous or liquid monomer
, often containing a vinyl
group, in order to initiate polymerization
. Polymers formed from this technique are generally highly branched and highly cross-linked, and adhere to solid surfaces well. The biggest advantage to this process is that polymers can be directly attached to a desired surface while the chains are growing, which reduces steps necessary for other coating
processes such as grafting
. This is very useful for pinhole-free coatings of 100 picometers to 1 micrometre thickness with solvent
insoluble polymers.
, with little attention being given to their properties. It was not until the 1960s that the properties of these polymers where found to be useful. It was found that flawless thin polymeric coatings could be formed on metals. By selecting the monomer type and the energy density
per monomer, known as the Yasuda parameter, the chemical composition and structure of the resulting thin film
can be varied with a wide range. These films are usually inert
, adhesive
, and have low dielectric constant
s. Some common monomers polymerized by this method include styrene, ethylene, methacrylate and pyridine, just to name a few. The 1970s brought about many advances in plasma polymerization, including the polymerization of many different types of monomers, and a probable mechanism
of the polymerization process being determined. Since this time most attention devoted to plasma polymerization has been in the fields of coatings, but since it is difficult to control polymer structure, it has very limited applications.
, and then lose energy through collision
s with neutral molecule
s in the gas phase. This leads to many chemically reactive species, which then lead to a plasma polymerization reaction. The electric discharge process for plasma polymerization is the “low-temperature plasma” method, because higher temperatures cause degradation
. These plasmas are formed by a direct current
, alternating current
or radio frequency
generator.
is shown in Figure 1. This reactor has internal electrode
s, and polymerization generally takes place on the cathode
side. All devices contain the thermostat
ic bath, which is used to regulate temperature, and a vacuum to regulate pressure.
Operation: The monomer gas comes into the Bell type reactor as a gaseous species, and then is put into the plasma state by the electrodes, in which the plasma may consist of radical
s, anions and cations. These monomers are then polymerized on the cathode surface, or some other surface placed in the apparatus by different mechanisms of which details are discussed below. The deposited polymers then propagate
off the surface and form growing chains with seemingly uniform consistency.
Another popular reactor type is the flow through reactor (continuous flow reactor), which also has internal electrodes, but this reactor allows monomer gas to flow through the reaction chamber as its name implies, which should give a more even coating for polymer film deposition. It has the advantage that more monomer keeps flowing into the reactor in order to deposit more polymer. It has the disadvantage of forming what is called “tail flame,” which is when polymerization extends into the vacuum line.
A third popular type of reactor is the electrodeless. This uses an RF coil wrapped around the glass apparatus, which then uses a radio frequency generator to form the plasma inside of the housing without the use of direct electrodes. The polymer can then be deposited as it is pushed through this RF coil toward the vacuum end of the apparatus. This has the advantage of not having polymer building up on the electrode surface, which is desirable when polymerizing onto other surfaces.
A fourth type of system growing in popularity is the atmospheric-pressure plasma
system, which is useful for depositing thin polymer films. This system bypasses the requirements for special hardware involving vacuums, which then makes it favorable for integrated industrial use. It has been shown that that polymers formed at atmospheric-pressure can have similar properties for coatings as those found in the low-pressure systems.
energy of 1–10 eV is required, with electron densities of 109 to 1012 per cubic centimeter, in order to form the desired plasma state. The formation of a low-temperature plasma is important; the electron temperatures are not equal to the gas temperatures and have a ratio of Te/Tg of 10 to 100, so that this process can occur at near ambient temperatures, which is advantageous because polymers degrade at high temperatures, so if a high-temperature plasma was used the polymers would degrade after formation or would never be formed. This entails non-equilibrium plasmas, which means that charged monomer species have more kinetic energy than neutral monomer species, and cause the transfer of energy to a substrate instead of an uncharged monomer.
rate of these reactions depends mostly on the monomer gas, which must be either gaseous or vaporized. However, other parameters are also important as well, such as power
, pressure
, flow rate, frequency
, electrode gap and reactor configuration. Low flow rates usually only depend on the amount of reactive species present for polymerization, whereas high flow rates depend on the amount of time that is spent in the reactor. Therefore, the maximum rate of polymerization is somewhere in the middle.
The fastest reactions tend to be in the order of triple-bonded > double-bonded > single bonded molecules, and also lower molecular weight molecules are faster than higher ones. So acetylene
is faster than ethylene
, and ethylene is faster than propene, etc.. The molecular weight factor in polymer deposition is dependent on the monomer flow rate, in which a higher molecular weight monomer typically near 200 g/mol needs a much higher flow rate of 15 g/cm2, whereas lower molecular weights around 50 g/mol require a flow rate of only 5 g/cm2. A heavy monomer therefore needs a faster flow, and would likely lead to increased pressures, decreasing polymerization rates.
Increased pressure tends to decrease polymerization rates reducing uniformity of deposition since uniformity is controlled by constant pressure. This is a reason that high-pressure plasma or atmospheric-pressure plasma
s are not usually used in favor of low-pressure systems, however atmospheric-pressure systems are still becoming more popular due to their ease of incorporation in manufacturing systems, with similar results now being formed compared to low pressure systems. At pressures greater than 1 torr
, oligomers are formed on the electrode surface, and the monomers also on the surface can dissolve them to get a low degree of polymerization
forming an oily substance. At low pressures, the reactive surfaces are low in monomer and facilitate growing high molecular weight polymers.
The rate of polymerization depends on input
power, until power saturation
occurs and the rate becomes independent of it. A narrower electrode gap also tends to increase polymerization rates because a higher electron density
per unit area is formed. Polymerization rates also depend on the type of apparatus used for the process. In general, increasing the frequency of alternating current glow discharge up to about 5 kHz increases the rate due to the formation of more free radicals. After this frequency, inertial effects of colliding monomers inhibit polymerization. This forms the first plateau for polymerization frequencies. A second maximum in frequency occurs at 6 MHz, where side reactions are overcome again and the reaction occurs through free radicals diffuse
d from plasma to the electrodes, at which point a second plateau is obtained. These parameters differ slightly for each monomer and must be optimized in-situ.
, since in a direct current system polymerization occurs mainly on the cathode. However, more investigation has led to the belief that the mechanism is more of a radical polymerization
process, since radicals tend to be trapped in the films, and termination can be overcome by reinitiation of oligomers. Other kinetic studies also support this theory.
In polymerization, both gas phase and surface reactions occur, but mechanism differs between high and low frequencies. At high frequencies it occurs in radical intermediates, whereas at low frequencies polymerization happens mainly on surfaces. As polymerization occurs, the pressure inside the chamber decreases in a closed system, since gas phase monomers go to solid polymers. An example diagram of the ways that polymerization can take place is shown in Figure 2, wherein the most abundant pathway is shown in blue with double arrows, with side pathways shown in black. The ablation
occurs by gas formation during polymerization. Polymerization has two pathways, either the plasma state or plasma induced processes, which both lead to deposited polymer.
Polymers can be placed on many substrates other than the electrode surfaces, such as glass
, other organic polymers or metals, when either a surface is placed in front of the electrodes, or placed in the middle between them. The ability for them to build off of electrode surfaces is likely to be an electrostatic interaction, while on other surfaces covalent attachment is possible.
The actual polymerization is likely to take place through many ionic and radical processes which are initiated by plasma formed from the glow discharge. There are many propagating species present at any given time as shown in Figure 3, which shows two different pathways for the polymerization to take place. The first pathway is a monofunctionalization process, which means it is analogous to a standard free radical polymerization mechanism (M•). The second pathway refers to a difunctional mechanism, which by example may contain a cationic and a radical propagating center on the same monomer (•M•)(Parylene
polymerization), which means the polymer can grow in multiple directions by multiple methods off one species, such as a surface or other monomer. Therefore, we would have a very rapid step-growth polymerization
. In the diagram, Mx refers to the original monomer molecule or any of many dissociation products such as chlorine
, fluorine
and hydrogen
. The M• species refers to those that are activated and capable of participating in reactions to form new covalent bond
s. The •M• species refers to an activated difunctional monomer species. The subscripts i, j, and k show the sizes of the different species involved. Even though radicals represent the activated species, any ion or radical could be used in the polymerization. As can be seen here, plasma polymerization is a very complex process, with many parameters effecting everything from rate to chain length.
There are also other stipulations that exist. Yasuda et al. studied 28 monomers and found that those containing aromatic groups, silicon
, olefinic group or nitrogen
(NH, NH2, CN) were readily polymerizable, while those containing oxygen
, halides, aliphatic hydrocarbons and cyclic hydrocarbons where decomposed more readily. The latter compounds have more ablation or side reactions present, which inhibit stable polymer formation. It is also possible to incorporate N2, H2O, and CO into copolymers of styrene.
Plasma polymers can be thought of as a type of graft polymers since they are grown off of a substrate
. These polymers are known to form nearly uniform surface deposition, which is one of their desirable properties. Polymers formed from this process often cross-link and form branches due to the multiple propagating species present in the plasma. This often leads to very insoluble polymers, which gives an advantage to this process, since hyperbranched polymers can be deposited directly without solvent.
, polyhexafluoropropylene, polytetramethyltin, polyhexamethyldisiloxane, polytetramethyldisiloxane, polypyridine, polyfuran, and poly-2-methyloxazoline.
The following are listed in order of decreasing rate of polymerization: polystyrene
, polymethyl styrene, polycyclopentadiene, polyacrylate, polyethyl acrylate, polymethyl methacrylate, polyvinyl acetate
, polyisoprene, polyisobutene, and polyethylene
.
Nearly all polymers created by this method have excellent appearance, are clear, and are significantly cross-linked. Linear polymers are not formed readily by plasma polymerization methods based on propagating species. Many other polymers could be formed by this method.
The most significant difference between conventional polymers and plasma polymers is that plasma polymers do not contain regular repeating units. Due to the number of different propagating species present at any one time as discussed above, the resultant polymer chains are highly-branched and are randomly terminated with a high degree of cross-linking. An example of a proposed structure for plasma polymerized ethylene demonstrating a large extend of cross-linking and branching is shown in Figure 4.
All plasma polymers contain free radicals as well. The amount of free radicals present varies between polymers and is dependent on the chemical structure of the monomer. Because the formation of the trapped free radicals is tied to the growth mechanism of the plasma polymers, the overall properties of the polymers directly correlate to the number of free radicals.
Plasma polymers also contain an internal stress. If a thick layer (e.g. 1 µm) of a plasma polymer is deposited on a glass slide, the plasma polymer will buckle and frequently crack. The curling is attributed to an internal stress formed in the plasma polymer during the polymer deposition. The degree of curling is dependent on the monomer as well as the conditions of the plasma polymerization.
Most plasma polymers are insoluble and infusible. These properties are due to the large amount of cross-linking in the polymers, previously discussed. Consequently the kinetic path length for these polymers must be sufficiently long, so these properties can be controlled to a point.
The permeabilities of plasma polymers also differ greatly from those of conventional polymers. Because of the absence of large-scale segmental mobility and the high degree of cross-linking within the polymers, the permeation of small molecules does not strictly follow the typical mechanisms of “solution-diffusion” or molecular-level sieve for such small permeants. Really the permeability characteristics of plasma polymers falls between these two ideal cases.
A final common characteristic of plasma polymers is the adhesion ability. The specifics of the adhesion ability for a given plasma polymer, such as thickness and characteristics of the surface layer, again are particular for a given plasma polymer and few generalizations can be made.
A second advantage is the ease of application of the polymers as coatings versus conventional coating processes. While coating a substrate with conventional polymers requires a number of steps, plasma polymerization accomplishes all these in essentially a single step. This leads to a cleaner and ‘greener’ synthesis and coating process, since no solvent is needed during the polymer preparation and no cleaning of the resultant polymer is needed either. Another ‘green’ aspect of the synthesis is that no initiator is needed for the polymer preparation since reusable electrodes cause the reaction to proceed. The resultant polymer coatings also have a number of advantages over typical coatings. These advantages include being nearly pinhole free, highly dense, and that the thickness of the coating can easily be varied.
There are also a number of disadvantages relating to plasma polymerization versus conventional methods. The most significant disadvantage is the high cost of the process. A vacuum system is required for the polymerization, significantly increasing the set up price.
Another disadvantage is due to the complexity of plasma processes. Because of the complexity it is not easy to achieve a good control over the chemical composition of the surface after modification. The influence of process parameters on the chemical composition of the resultant polymer means it can take a long time to determine the optimal conditions. The complexity of the process also makes it impossible to theorize what the resultant polymer will look like, unlike conventional polymers which can be easily determined based on the monomer.
s, protective coatings, printing
, membranes, biomedical applications and so on have all been studied.
A significant area of research has been on the use of plasma polymer films as permeation
membranes. The permeability characteristics of plasma polymers deposited on porous substrates are different than usual polymer films. The characteristics depend on the deposition and polymerization mechanism. Plasma polymers as membranes for separation of oxygen and nitrogen, ethanol and water, and water vapor permeation have all been studied. The application of plasma polymerized thin films as reverse osmosis
membranes has received considerable attention as well. Yasuda et al. have shown membranes prepared with plasma polymerization made from nitrogen containing monomers can yield up to 98% salt rejection with a flux
of 6.4 gallons/ft2 a day. Further research has shown that varying the monomers of the membrane offer other properties as well, such as chlorine resistance.
Plasma-polymerized films have also found electrical applications. Given that plasma polymers frequently contain many polar
groups, which form when the radicals react with oxygen in air during the polymerization process, the plasma polymers were expected to be good dielectric materials in thin film form. Studies have shown that the plasma polymers generally do in fact have a higher dielectric property. Some plasma polymers have been applied as chemical sensory devices due to their electrical properties. Plasma polymers have been studied as chemical sensory devices for humidity, propane, and carbon dioxide amongst others. Thus far issues with instability against aging and humidity have limited their commercial applications.
The application of plasma polymers as coatings has also been studied. Plasma polymers formed from tetramethoxysilane have been studied as protective coatings and have shown to increase the hardness of polyethylene
and polycarbonate
. The use of plasma polymers to coat plastic
lens
es is increasing in popularity. Plasma depositions are able to easily coat curved materials with a good uniformity, such as those of bifocals
. The different plasma polymers used can be not only scratch resistant, but also hydrophobic leading to anti-fogging effects.
Fragment
Fragment may refer to:* A small part/portion broken off something; debris* Fragment , all the data necessary to generate a pixel in the frame buffer* Sentence fragment, a sentence not containing a subject or a predicate...
gaseous or liquid monomer
Monomer
A monomer is an atom or a small molecule that may bind chemically to other monomers to form a polymer; the term "monomeric protein" may also be used to describe one of the proteins making up a multiprotein complex...
, often containing a vinyl
Vinyl
A vinyl compound is any organic compound that contains a vinyl group ,which are derivatives of ethene, CH2=CH2, with one hydrogen atom replaced with some other group...
group, in order to initiate polymerization
Polymerization
In polymer chemistry, polymerization is a process of reacting monomer molecules together in a chemical reaction to form three-dimensional networks or polymer chains...
. Polymers formed from this technique are generally highly branched and highly cross-linked, and adhere to solid surfaces well. The biggest advantage to this process is that polymers can be directly attached to a desired surface while the chains are growing, which reduces steps necessary for other coating
Coating
Coating is a covering that is applied to the surface of an object, usually referred to as the substrate. In many cases coatings are applied to improve surface properties of the substrate, such as appearance, adhesion, wetability, corrosion resistance, wear resistance, and scratch resistance...
processes such as grafting
Grafting
Grafting is a horticultural technique whereby tissues from one plant are inserted into those of another so that the two sets of vascular tissues may join together. This vascular joining is called inosculation...
. This is very useful for pinhole-free coatings of 100 picometers to 1 micrometre thickness with solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
insoluble polymers.
Introduction
In as early as the 1870s “polymers” formed by this process were known, but these polymers were initially thought of as undesirable byproducts associated with electric dischargeElectric discharge
Electric discharge describes any flow of electric charge through a gas, liquid or solid. Electric discharges include:*Electric glow discharge*Electric arc*Electrostatic discharge*Electric discharge in gases*Leader *Partial discharge...
, with little attention being given to their properties. It was not until the 1960s that the properties of these polymers where found to be useful. It was found that flawless thin polymeric coatings could be formed on metals. By selecting the monomer type and the energy density
Energy density
Energy density is a term used for the amount of energy stored in a given system or region of space per unit volume. Often only the useful or extractable energy is quantified, which is to say that chemically inaccessible energy such as rest mass energy is ignored...
per monomer, known as the Yasuda parameter, the chemical composition and structure of the resulting thin film
Thin film
A thin film is a layer of material ranging from fractions of a nanometer to several micrometers in thickness. Electronic semiconductor devices and optical coatings are the main applications benefiting from thin film construction....
can be varied with a wide range. These films are usually inert
Inert
-Chemistry:In chemistry, the term inert is used to describe a substance that is not chemically reactive.The noble gases were previously known as inert gases because of their perceived lack of participation in any chemical reactions...
, adhesive
Adhesive
An adhesive, or glue, is a mixture in a liquid or semi-liquid state that adheres or bonds items together. Adhesives may come from either natural or synthetic sources. The types of materials that can be bonded are vast but they are especially useful for bonding thin materials...
, and have low dielectric constant
Dielectric constant
The relative permittivity of a material under given conditions reflects the extent to which it concentrates electrostatic lines of flux. In technical terms, it is the ratio of the amount of electrical energy stored in a material by an applied voltage, relative to that stored in a vacuum...
s. Some common monomers polymerized by this method include styrene, ethylene, methacrylate and pyridine, just to name a few. The 1970s brought about many advances in plasma polymerization, including the polymerization of many different types of monomers, and a probable mechanism
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
of the polymerization process being determined. Since this time most attention devoted to plasma polymerization has been in the fields of coatings, but since it is difficult to control polymer structure, it has very limited applications.
Basic operating mechanism
Glow discharge
Glow discharge is a technique in polymerization which forms free electrons which gain energy from an electric fieldElectric field
In physics, an electric field surrounds electrically charged particles and time-varying magnetic fields. The electric field depicts the force exerted on other electrically charged objects by the electrically charged particle the field is surrounding...
, and then lose energy through collision
Collision
A collision is an isolated event which two or more moving bodies exert forces on each other for a relatively short time.Although the most common colloquial use of the word "collision" refers to accidents in which two or more objects collide, the scientific use of the word "collision" implies...
s with neutral molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
s in the gas phase. This leads to many chemically reactive species, which then lead to a plasma polymerization reaction. The electric discharge process for plasma polymerization is the “low-temperature plasma” method, because higher temperatures cause degradation
Chemical decomposition
Chemical decomposition, analysis or breakdown is the separation of a chemical compound into elements or simpler compounds. It is sometimes defined as the exact opposite of a chemical synthesis. Chemical decomposition is often an undesired chemical reaction...
. These plasmas are formed by a direct current
Direct current
Direct current is the unidirectional flow of electric charge. Direct current is produced by such sources as batteries, thermocouples, solar cells, and commutator-type electric machines of the dynamo type. Direct current may flow in a conductor such as a wire, but can also flow through...
, alternating current
Alternating current
In alternating current the movement of electric charge periodically reverses direction. In direct current , the flow of electric charge is only in one direction....
or radio frequency
Radio frequency
Radio frequency is a rate of oscillation in the range of about 3 kHz to 300 GHz, which corresponds to the frequency of radio waves, and the alternating currents which carry radio signals...
generator.
Types of reactors
There are a few designs for apparatus used in plasma polymerization, one of which is the Bell (static type), in which monomer gas is put into the reaction chamber, but does not flow through the chamber. It comes in and polymerizes without removal. This type of reactorChemical reactor
In chemical engineering, chemical reactors are vessels designed to contain chemical reactions. The design of a chemical reactor deals with multiple aspects of chemical engineering. Chemical engineers design reactors to maximize net present value for the given reaction...
is shown in Figure 1. This reactor has internal electrode
Electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit...
s, and polymerization generally takes place on the cathode
Cathode
A cathode is an electrode through which electric current flows out of a polarized electrical device. Mnemonic: CCD .Cathode polarity is not always negative...
side. All devices contain the thermostat
Thermostat
A thermostat is the component of a control system which regulates the temperature of a system so that the system's temperature is maintained near a desired setpoint temperature. The thermostat does this by switching heating or cooling devices on or off, or regulating the flow of a heat transfer...
ic bath, which is used to regulate temperature, and a vacuum to regulate pressure.
Operation: The monomer gas comes into the Bell type reactor as a gaseous species, and then is put into the plasma state by the electrodes, in which the plasma may consist of radical
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
s, anions and cations. These monomers are then polymerized on the cathode surface, or some other surface placed in the apparatus by different mechanisms of which details are discussed below. The deposited polymers then propagate
Wave propagation
Wave propagation is any of the ways in which waves travel.With respect to the direction of the oscillation relative to the propagation direction, we can distinguish between longitudinal wave and transverse waves....
off the surface and form growing chains with seemingly uniform consistency.
Another popular reactor type is the flow through reactor (continuous flow reactor), which also has internal electrodes, but this reactor allows monomer gas to flow through the reaction chamber as its name implies, which should give a more even coating for polymer film deposition. It has the advantage that more monomer keeps flowing into the reactor in order to deposit more polymer. It has the disadvantage of forming what is called “tail flame,” which is when polymerization extends into the vacuum line.
A third popular type of reactor is the electrodeless. This uses an RF coil wrapped around the glass apparatus, which then uses a radio frequency generator to form the plasma inside of the housing without the use of direct electrodes. The polymer can then be deposited as it is pushed through this RF coil toward the vacuum end of the apparatus. This has the advantage of not having polymer building up on the electrode surface, which is desirable when polymerizing onto other surfaces.
A fourth type of system growing in popularity is the atmospheric-pressure plasma
Atmospheric-pressure plasma
Atmospheric-pressure plasma is the name given to the special case of a plasma in which the pressure approximately matches that of the surrounding atmosphere – the so-called normal pressure....
system, which is useful for depositing thin polymer films. This system bypasses the requirements for special hardware involving vacuums, which then makes it favorable for integrated industrial use. It has been shown that that polymers formed at atmospheric-pressure can have similar properties for coatings as those found in the low-pressure systems.
Physical process characteristics
The formation of a plasma for polymerization depends on many of the following. An electronElectron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
energy of 1–10 eV is required, with electron densities of 109 to 1012 per cubic centimeter, in order to form the desired plasma state. The formation of a low-temperature plasma is important; the electron temperatures are not equal to the gas temperatures and have a ratio of Te/Tg of 10 to 100, so that this process can occur at near ambient temperatures, which is advantageous because polymers degrade at high temperatures, so if a high-temperature plasma was used the polymers would degrade after formation or would never be formed. This entails non-equilibrium plasmas, which means that charged monomer species have more kinetic energy than neutral monomer species, and cause the transfer of energy to a substrate instead of an uncharged monomer.
Kinetics
The kineticKinetic
-Art and entertainment:* Kinetic * Kinetic art* The 13th episode of the first season of the TV series Smallville* Kinetic, a comic by Allan Heinberg and Kelley Pucklett-Companies:...
rate of these reactions depends mostly on the monomer gas, which must be either gaseous or vaporized. However, other parameters are also important as well, such as power
Power (physics)
In physics, power is the rate at which energy is transferred, used, or transformed. For example, the rate at which a light bulb transforms electrical energy into heat and light is measured in watts—the more wattage, the more power, or equivalently the more electrical energy is used per unit...
, pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
, flow rate, frequency
Frequency
Frequency is the number of occurrences of a repeating event per unit time. It is also referred to as temporal frequency.The period is the duration of one cycle in a repeating event, so the period is the reciprocal of the frequency...
, electrode gap and reactor configuration. Low flow rates usually only depend on the amount of reactive species present for polymerization, whereas high flow rates depend on the amount of time that is spent in the reactor. Therefore, the maximum rate of polymerization is somewhere in the middle.
The fastest reactions tend to be in the order of triple-bonded > double-bonded > single bonded molecules, and also lower molecular weight molecules are faster than higher ones. So acetylene
Acetylene
Acetylene is the chemical compound with the formula C2H2. It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in pure form and thus is usually handled as a solution.As an alkyne, acetylene is unsaturated because...
is faster than ethylene
Ethylene
Ethylene is a gaseous organic compound with the formula . It is the simplest alkene . Because it contains a carbon-carbon double bond, ethylene is classified as an unsaturated hydrocarbon. Ethylene is widely used in industry and is also a plant hormone...
, and ethylene is faster than propene, etc.. The molecular weight factor in polymer deposition is dependent on the monomer flow rate, in which a higher molecular weight monomer typically near 200 g/mol needs a much higher flow rate of 15 g/cm2, whereas lower molecular weights around 50 g/mol require a flow rate of only 5 g/cm2. A heavy monomer therefore needs a faster flow, and would likely lead to increased pressures, decreasing polymerization rates.
Increased pressure tends to decrease polymerization rates reducing uniformity of deposition since uniformity is controlled by constant pressure. This is a reason that high-pressure plasma or atmospheric-pressure plasma
Atmospheric-pressure plasma
Atmospheric-pressure plasma is the name given to the special case of a plasma in which the pressure approximately matches that of the surrounding atmosphere – the so-called normal pressure....
s are not usually used in favor of low-pressure systems, however atmospheric-pressure systems are still becoming more popular due to their ease of incorporation in manufacturing systems, with similar results now being formed compared to low pressure systems. At pressures greater than 1 torr
Torr
The torr is a non-SI unit of pressure with the ratio of 760 to 1 standard atmosphere, chosen to be roughly equal to the fluid pressure exerted by a millimetre of mercury, i.e., a pressure of 1 torr is approximately equal to 1 mmHg...
, oligomers are formed on the electrode surface, and the monomers also on the surface can dissolve them to get a low degree of polymerization
Degree of polymerization
The degree of polymerization, or DP, is usually defined as the number of monomeric units in a macromolecule or polymer or oligomer molecule.For a homopolymer, there is only one type of monomeric unit andthe number-average degree of polymerization is given by...
forming an oily substance. At low pressures, the reactive surfaces are low in monomer and facilitate growing high molecular weight polymers.
The rate of polymerization depends on input
Input
Input is the term denoting either an entrance or changes which are inserted into a system and which activate/modify a process. It is an abstract concept, used in the modeling, system design and system exploitation...
power, until power saturation
Saturation
Saturation or saturated may refer to:- Meteorology :* Dew point, which is a temperature that occurs when atmospheric humidity reaches 100% and the air is saturated with moisture- Physics :...
occurs and the rate becomes independent of it. A narrower electrode gap also tends to increase polymerization rates because a higher electron density
Electron density
Electron density is the measure of the probability of an electron being present at a specific location.In molecules, regions of electron density are usually found around the atom, and its bonds...
per unit area is formed. Polymerization rates also depend on the type of apparatus used for the process. In general, increasing the frequency of alternating current glow discharge up to about 5 kHz increases the rate due to the formation of more free radicals. After this frequency, inertial effects of colliding monomers inhibit polymerization. This forms the first plateau for polymerization frequencies. A second maximum in frequency occurs at 6 MHz, where side reactions are overcome again and the reaction occurs through free radicals diffuse
Diffusion
Molecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
d from plasma to the electrodes, at which point a second plateau is obtained. These parameters differ slightly for each monomer and must be optimized in-situ.
Synthetic routes
Plasma contains many species such as ions, free radicals and electrons, so it is important to look at what contributes to the polymerization process most. The first suggested process by Westwood et al. was that of a cationic polymerizationCationic polymerization
Cationic polymerization is a type of chain growth polymerization in which a cationic initiator transfers charge to a monomer which becomes reactive. This reactive monomer goes on to react similarly with other monomers to form a polymer...
, since in a direct current system polymerization occurs mainly on the cathode. However, more investigation has led to the belief that the mechanism is more of a radical polymerization
Radical polymerization
Free radical polymerization is a method of polymerization by which a polymer forms by the successive addition of free radical building blocks. Free radicals can be formed via a number of different mechanisms usually involving separate initiator molecules...
process, since radicals tend to be trapped in the films, and termination can be overcome by reinitiation of oligomers. Other kinetic studies also support this theory.
In polymerization, both gas phase and surface reactions occur, but mechanism differs between high and low frequencies. At high frequencies it occurs in radical intermediates, whereas at low frequencies polymerization happens mainly on surfaces. As polymerization occurs, the pressure inside the chamber decreases in a closed system, since gas phase monomers go to solid polymers. An example diagram of the ways that polymerization can take place is shown in Figure 2, wherein the most abundant pathway is shown in blue with double arrows, with side pathways shown in black. The ablation
Ablation
Ablation is removal of material from the surface of an object by vaporization, chipping, or other erosive processes. This occurs in spaceflight during ascent and atmospheric reentry, glaciology, medicine, and passive fire protection.-Spaceflight:...
occurs by gas formation during polymerization. Polymerization has two pathways, either the plasma state or plasma induced processes, which both lead to deposited polymer.
Polymers can be placed on many substrates other than the electrode surfaces, such as glass
Glass
Glass is an amorphous solid material. Glasses are typically brittle and optically transparent.The most familiar type of glass, used for centuries in windows and drinking vessels, is soda-lime glass, composed of about 75% silica plus Na2O, CaO, and several minor additives...
, other organic polymers or metals, when either a surface is placed in front of the electrodes, or placed in the middle between them. The ability for them to build off of electrode surfaces is likely to be an electrostatic interaction, while on other surfaces covalent attachment is possible.
The actual polymerization is likely to take place through many ionic and radical processes which are initiated by plasma formed from the glow discharge. There are many propagating species present at any given time as shown in Figure 3, which shows two different pathways for the polymerization to take place. The first pathway is a monofunctionalization process, which means it is analogous to a standard free radical polymerization mechanism (M•). The second pathway refers to a difunctional mechanism, which by example may contain a cationic and a radical propagating center on the same monomer (•M•)(Parylene
Parylene
Parylene is the tradename for a variety of chemical vapor deposited poly polymers used as moisture and dielectric barriers. Among them, Parylene C is the most popular due to its combination of barrier properties, cost, and other processing advantages.Parylene is green polymer chemistry...
polymerization), which means the polymer can grow in multiple directions by multiple methods off one species, such as a surface or other monomer. Therefore, we would have a very rapid step-growth polymerization
Step-growth polymerization
Step-growth polymerization refers to a type of polymerization mechanism in which bi-functional or multifunctional monomers react to form first dimers, then trimers, longer oligomers and eventually long chain polymers. Many naturally occurring and some synthetic polymers are produced by step-growth...
. In the diagram, Mx refers to the original monomer molecule or any of many dissociation products such as chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
, fluorine
Fluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
and hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
. The M• species refers to those that are activated and capable of participating in reactions to form new covalent bond
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
s. The •M• species refers to an activated difunctional monomer species. The subscripts i, j, and k show the sizes of the different species involved. Even though radicals represent the activated species, any ion or radical could be used in the polymerization. As can be seen here, plasma polymerization is a very complex process, with many parameters effecting everything from rate to chain length.
Common monomers/polymers
| Name | Structure |
|---|---|
| Thiophene Thiophene Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a flat five-membered ring, it is aromatic as indicated by its extensive substitution reactions. Related to thiophene are benzothiophene and dibenzothiophene, containing the thiophene ring fused with one and two benzene... |
|
| Pyridine Pyridine Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom... |
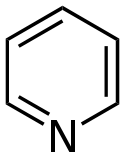 |
| Acrylonitrile Acrylonitrile Acrylonitrile is the chemical compound with the formula C3H3N. This pungent-smelling colorless liquid often appears yellow due to impurities. It is an important monomer for the manufacture of useful plastics. In terms of its molecular structure, it consists of a vinyl group linked to a nitrile... |
|
| Furan Furan Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen. The class of compounds containing such rings are also referred to as furans.... |
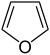 |
| Styrene | |
| Acetylene Acetylene Acetylene is the chemical compound with the formula C2H2. It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in pure form and thus is usually handled as a solution.As an alkyne, acetylene is unsaturated because... |
|
| 2-Methyloxazoline | |
| Tetramethyldisiloxane |
Monomers
As can be seen in the monomer table, many simple monomers are readily polymerized by this method, but most must be smaller ionizable species because they have to be able to go into the plasma state. It must be noted that though monomers with multiple bonds polymerize readily, it is not a necessary requirement, as ethane, silicones and many others polymerize also.There are also other stipulations that exist. Yasuda et al. studied 28 monomers and found that those containing aromatic groups, silicon
Silicon
Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table...
, olefinic group or nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
(NH, NH2, CN) were readily polymerizable, while those containing oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
, halides, aliphatic hydrocarbons and cyclic hydrocarbons where decomposed more readily. The latter compounds have more ablation or side reactions present, which inhibit stable polymer formation. It is also possible to incorporate N2, H2O, and CO into copolymers of styrene.
Plasma polymers can be thought of as a type of graft polymers since they are grown off of a substrate
Substrate
Substrate may mean:*Substrate , Natural stone, masonry surface, ceramic and porcelain tiles*Substrate , the material used in the bottom of an aquarium*Substrate , the material used in the bottom of a vivarium or terrarium...
. These polymers are known to form nearly uniform surface deposition, which is one of their desirable properties. Polymers formed from this process often cross-link and form branches due to the multiple propagating species present in the plasma. This often leads to very insoluble polymers, which gives an advantage to this process, since hyperbranched polymers can be deposited directly without solvent.
Polymers
Common polymers include: polythiophenePolythiophene
Polythiophenes result from the polymerization of thiophenes, a sulfur heterocycle, that can become conducting when electrons are added or removed from the conjugated π-orbitals via doping....
, polyhexafluoropropylene, polytetramethyltin, polyhexamethyldisiloxane, polytetramethyldisiloxane, polypyridine, polyfuran, and poly-2-methyloxazoline.
The following are listed in order of decreasing rate of polymerization: polystyrene
Polystyrene
Polystyrene ) also known as Thermocole, abbreviated following ISO Standard PS, is an aromatic polymer made from the monomer styrene, a liquid hydrocarbon that is manufactured from petroleum by the chemical industry...
, polymethyl styrene, polycyclopentadiene, polyacrylate, polyethyl acrylate, polymethyl methacrylate, polyvinyl acetate
Polyvinyl acetate
Polyvinyl acetate, PVA, PVAc, poly, is a rubbery synthetic polymer with the formula n. It belongs to the polyvinyl esters family with the general formula -[RCOOCHCH2]-...
, polyisoprene, polyisobutene, and polyethylene
Polyethylene
Polyethylene or polythene is the most widely used plastic, with an annual production of approximately 80 million metric tons...
.
Nearly all polymers created by this method have excellent appearance, are clear, and are significantly cross-linked. Linear polymers are not formed readily by plasma polymerization methods based on propagating species. Many other polymers could be formed by this method.
General characteristics of plasma polymers
The properties of plasma polymers differ greatly from those of conventional polymers. While both types are dependent on the chemical properties of the monomer, the properties of plasma polymers depend more greatly on the design of the reactor and the chemical and physical characteristics of the substrate on which the plasma polymer is deposited. The location within the reactor where the deposition occurs also has an effect on the resultant polymer's properties. In fact by using plasma polymerization with a single monomer and varying the reactor, substrate, etc. a variety of polymers, each having different physical and chemical properties, can be prepared. The large dependence of the polymer features on these factors make it difficult to assign a set of basic characteristics, but a few common properties that set plasma polymers apart from conventional polymers do exist.The most significant difference between conventional polymers and plasma polymers is that plasma polymers do not contain regular repeating units. Due to the number of different propagating species present at any one time as discussed above, the resultant polymer chains are highly-branched and are randomly terminated with a high degree of cross-linking. An example of a proposed structure for plasma polymerized ethylene demonstrating a large extend of cross-linking and branching is shown in Figure 4.
All plasma polymers contain free radicals as well. The amount of free radicals present varies between polymers and is dependent on the chemical structure of the monomer. Because the formation of the trapped free radicals is tied to the growth mechanism of the plasma polymers, the overall properties of the polymers directly correlate to the number of free radicals.
Plasma polymers also contain an internal stress. If a thick layer (e.g. 1 µm) of a plasma polymer is deposited on a glass slide, the plasma polymer will buckle and frequently crack. The curling is attributed to an internal stress formed in the plasma polymer during the polymer deposition. The degree of curling is dependent on the monomer as well as the conditions of the plasma polymerization.
Most plasma polymers are insoluble and infusible. These properties are due to the large amount of cross-linking in the polymers, previously discussed. Consequently the kinetic path length for these polymers must be sufficiently long, so these properties can be controlled to a point.
The permeabilities of plasma polymers also differ greatly from those of conventional polymers. Because of the absence of large-scale segmental mobility and the high degree of cross-linking within the polymers, the permeation of small molecules does not strictly follow the typical mechanisms of “solution-diffusion” or molecular-level sieve for such small permeants. Really the permeability characteristics of plasma polymers falls between these two ideal cases.
A final common characteristic of plasma polymers is the adhesion ability. The specifics of the adhesion ability for a given plasma polymer, such as thickness and characteristics of the surface layer, again are particular for a given plasma polymer and few generalizations can be made.
Advantages and disadvantages
Plasma polymerization offers a number of advantages over other polymerization methods and in general. The most significant advantage of plasma polymerization is its ability to produce polymer films of organic compounds that do not polymerize under normal chemical polymerization conditions. Nearly all monomers, even saturated hydrocarbons and organic compounds without a polymerizable structure such as a double bond, can be polymerized with this technique.A second advantage is the ease of application of the polymers as coatings versus conventional coating processes. While coating a substrate with conventional polymers requires a number of steps, plasma polymerization accomplishes all these in essentially a single step. This leads to a cleaner and ‘greener’ synthesis and coating process, since no solvent is needed during the polymer preparation and no cleaning of the resultant polymer is needed either. Another ‘green’ aspect of the synthesis is that no initiator is needed for the polymer preparation since reusable electrodes cause the reaction to proceed. The resultant polymer coatings also have a number of advantages over typical coatings. These advantages include being nearly pinhole free, highly dense, and that the thickness of the coating can easily be varied.
There are also a number of disadvantages relating to plasma polymerization versus conventional methods. The most significant disadvantage is the high cost of the process. A vacuum system is required for the polymerization, significantly increasing the set up price.
Another disadvantage is due to the complexity of plasma processes. Because of the complexity it is not easy to achieve a good control over the chemical composition of the surface after modification. The influence of process parameters on the chemical composition of the resultant polymer means it can take a long time to determine the optimal conditions. The complexity of the process also makes it impossible to theorize what the resultant polymer will look like, unlike conventional polymers which can be easily determined based on the monomer.
Applications
The advantages offered by plasma polymerization have resulted in substantial research on the applications of these polymers. The vastly different chemical and mechanical properties offered by polymers formed with plasma polymerization means they can be applied to countless different systems. Applications ranging from adhesion, composite materialComposite material
Composite materials, often shortened to composites or called composition materials, are engineered or naturally occurring materials made from two or more constituent materials with significantly different physical or chemical properties which remain separate and distinct at the macroscopic or...
s, protective coatings, printing
Printing
Printing is a process for reproducing text and image, typically with ink on paper using a printing press. It is often carried out as a large-scale industrial process, and is an essential part of publishing and transaction printing....
, membranes, biomedical applications and so on have all been studied.
A significant area of research has been on the use of plasma polymer films as permeation
Permeation
Permeation, in physics and engineering, is the penetration of a permeate through a solid, and is related to a material's intrinsic permeability...
membranes. The permeability characteristics of plasma polymers deposited on porous substrates are different than usual polymer films. The characteristics depend on the deposition and polymerization mechanism. Plasma polymers as membranes for separation of oxygen and nitrogen, ethanol and water, and water vapor permeation have all been studied. The application of plasma polymerized thin films as reverse osmosis
Reverse osmosis
Reverse osmosis is a membrane technical filtration method that removes many types of large molecules and ions from solutions by applying pressure to the solution when it is on one side of a selective membrane. The result is that the solute is retained on the pressurized side of the membrane and...
membranes has received considerable attention as well. Yasuda et al. have shown membranes prepared with plasma polymerization made from nitrogen containing monomers can yield up to 98% salt rejection with a flux
Flux
In the various subfields of physics, there exist two common usages of the term flux, both with rigorous mathematical frameworks.* In the study of transport phenomena , flux is defined as flow per unit area, where flow is the movement of some quantity per time...
of 6.4 gallons/ft2 a day. Further research has shown that varying the monomers of the membrane offer other properties as well, such as chlorine resistance.
Plasma-polymerized films have also found electrical applications. Given that plasma polymers frequently contain many polar
Chemical polarity
In chemistry, polarity refers to a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Molecular polarity is dependent on the difference in...
groups, which form when the radicals react with oxygen in air during the polymerization process, the plasma polymers were expected to be good dielectric materials in thin film form. Studies have shown that the plasma polymers generally do in fact have a higher dielectric property. Some plasma polymers have been applied as chemical sensory devices due to their electrical properties. Plasma polymers have been studied as chemical sensory devices for humidity, propane, and carbon dioxide amongst others. Thus far issues with instability against aging and humidity have limited their commercial applications.
The application of plasma polymers as coatings has also been studied. Plasma polymers formed from tetramethoxysilane have been studied as protective coatings and have shown to increase the hardness of polyethylene
Polyethylene
Polyethylene or polythene is the most widely used plastic, with an annual production of approximately 80 million metric tons...
and polycarbonate
Polycarbonate
PolycarbonatePhysical PropertiesDensity 1.20–1.22 g/cm3Abbe number 34.0Refractive index 1.584–1.586FlammabilityV0-V2Limiting oxygen index25–27%Water absorption – Equilibrium0.16–0.35%Water absorption – over 24 hours0.1%...
. The use of plasma polymers to coat plastic
Plastic
A plastic material is any of a wide range of synthetic or semi-synthetic organic solids used in the manufacture of industrial products. Plastics are typically polymers of high molecular mass, and may contain other substances to improve performance and/or reduce production costs...
lens
Lens (optics)
A lens is an optical device with perfect or approximate axial symmetry which transmits and refracts light, converging or diverging the beam. A simple lens consists of a single optical element...
es is increasing in popularity. Plasma depositions are able to easily coat curved materials with a good uniformity, such as those of bifocals
Bifocals
Bifocals are eyeglasses with two distinct optical powers. Bifocals are most commonly prescribed to people with presbyopia who also require a correction for myopia, hyperopia, and/or astigmatism.-History:...
. The different plasma polymers used can be not only scratch resistant, but also hydrophobic leading to anti-fogging effects.

