
Polymer degradation
Encyclopedia
Polymer degradation is a change in the properties—tensile strength
, colour, shape, etc.—of a polymer
or polymer-based product under the influence of one or more environmental factors such as heat
, light
or chemicals such as acid
s, alkali
s and some salts. These changes are usually undesirable, such as cracking and chemical disintegration of products or, more rarely, desirable, as in biodegradation
, or deliberately lowering the molecular weight of a polymer for recycling
. The changes in properties are often termed "aging".
In a finished product such a change is to be prevented or delayed. Degradation can be useful for recycling
/reusing the polymer waste to prevent or reduce environmental pollution
. Degradation can also be induced deliberately to assist structure determination.
Polymeric molecule
s are very large (on the molecular scale), and their unique and useful properties are mainly a result of their size. Any loss in chain length lowers tensile strength and is a primary cause of premature cracking.
, polypropylene
, polyvinyl chloride
, polyethylene terephthalate
, polystyrene
, polycarbonate
, and poly(methyl methacrylate) (Plexiglass). These make up nearly 98% of all polymers and plastics encountered in daily life. Each of these polymers has its own characteristic modes of degradation and resistances to heat, light and chemicals. Polyethylene, polypropylene, and poly(methyl methacrylate) are sensitive to oxidation and UV radiation, while PVC may discolour at high temperatures due to loss of hydrogen chloride
gas, and become very brittle. PET is sensitive to hydrolysis
and attack by strong acid
s, while polycarbonate depolymerizes rapidly when exposed to strong alkali
s.
For example, polyethylene usually degrades by random scission - that is by a random breakage of the linkages (bonds) that hold the atoms of the polymer together. When this polymer is heated above 450 Celsius
it becomes a complex mixture of molecules of various sizes that resemble gasoline. Other polymers - like polyalphamethylstyrene - undergo 'unspecific' chain scission with breakage occurring only at the ends; they literally unzip or depolymerize to become the constituent monomers.


s and gamma ray
s are usually involved in such reactions.
s, polyamide
s and polycarbonate
s can be degraded by solvolysis
and mainly hydrolysis
to give lower molecular weight molecules. The hydrolysis takes place in the presence of water containing an acid
or a base
as catalyst.
Polyamide
is sensitive to degradation by acids and polyamide mouldings will crack when attacked by strong acids. For example, the fracture surface of a fuel connector showed the progressive growth of the crack from acid attack (Ch) to the final cusp (C) of polymer. The problem is known as stress corrosion cracking
, and in this case was caused by hydrolysis
of the polymer. It was the reverse reaction of the synthesis of the polymer:
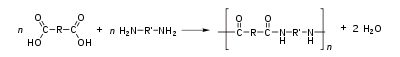
 Cracks can be formed in many different elastomers by ozone
Cracks can be formed in many different elastomers by ozone
attack. Tiny traces of the gas in the air will attack double bonds in rubber chains, with Natural rubber, polybutadiene
, Styrene-butadiene
rubber and NBR being most sensitive to degradation. Ozone cracks form in products under tension, but the critical strain is very small. The cracks are always oriented at right angles to the strain axis, so will form around the circumference in a rubber tube bent over. Such cracks are dangerous when they occur in fuel pipes because the cracks will grow from the outside exposed surfaces into the bore of the pipe, and fuel leakage and fire may follow. The problem of ozone cracking
can be prevented by adding anti-ozonants to the rubber before vulcanization
. Ozone cracks were commonly seen in automobile tire
sidewalls, but are now seen rarely thanks to these additives. On the other hand, the problem does recur in unprotected products such as rubber tubing and seals.
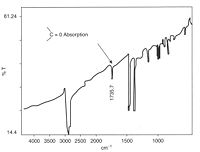 Polymers are susceptible to attack by atmospheric oxygen
Polymers are susceptible to attack by atmospheric oxygen
, especially at elevated temperatures encountered during processing to shape. Many process methods such as extrusion
and injection moulding
involve pumping molten polymer into tools, and the high temperatures needed for melting may result in oxidation unless precautions are taken. For example, a forearm crutch
suddenly snapped and the user was severely injured in the resulting fall. The crutch had fractured across a polypropylene
insert within the aluminium tube of the device, and infra-red spectroscopy of the material showed that it had oxidised, possible as a result of poor moulding.
Oxidation is usually relatively easy to detect owing to the strong absorption by the carbonyl group in the spectrum of polyolefins. Polypropylene
has a relatively simple spectrum with few peaks at the carbonyl position (like polyethylene
). Oxidation tends to start at tertiary carbon atoms because the free radicals formed here are more stable and longer lasting, making them more susceptible to attack by oxygen
. The carbonyl group can be further oxidised to break the chain, this weakens the material by lowering its molecular weight, and cracks start to grow in the regions affected.
(Cu) and iron
(Fe) are put into contact and then immersed in salt water, the iron will undergo corrosion
, or rust. This is called a galvanic circuit where the copper is the noble metal
and the iron is the active metal, i.e., the copper is the cathode
or positive (+) electrode
and the iron is the anode
, or negative (-) electrode
. A battery
is formed. It follows that plastics are made stronger by impregnating them with thin carbon fibers only a few micrometers in diameter known as carbon fiber
reinforced polymers (CFRP). This is to produce materials that are high strength and resistant to high temperatures. The carbon fibers act as a noble metal similar to gold (Au) or platinum (Pt). When put into contact with a more active metal, for example with aluminum (Al) in salt water the aluminum corrodes. However in early 1990, it was reported that imide-linked resins in CFRP composite
s degrade when bare composite is coupled with an active metal in salt water environments. This is because corrosion not only occurs at the aluminum anode
, but also at the carbon fiber
cathode
in the form of a very strong base with a pH
of about 13. This strong base reacts with the polymer chain structure degrading the polymer. Polymers affected include bismaleimides (BMI), condensation polyimide
s, triazine
s, and blends thereof. Degradation occurs in the form of dissolved resin and loose fibers. The hydroxyl ions generated at the graphite cathode
attack the O-C-N bond in the polyimide structure. Standard corrosion protection procedures were found to prevent polymer degradation under most conditions.
 Another highly reactive gas is chlorine
Another highly reactive gas is chlorine
, which will attack susceptible polymers such as acetal resin and polybutylene
pipework. There have been many examples of such pipes and acetal fittings failing in properties in the US as a result of chlorine-induced cracking. In essence, the gas attacks sensitive parts of the chain molecules (especially secondary, tertiary, or allylic carbon atoms), oxidizing the chains and ultimately causing chain cleavage. The root cause is traces of chlorine in the water supply, added for its anti-bacterial action, attack occurring even at parts per million traces of the dissolved gas. The chlorine attacks weak parts of a product, and in the case of an acetal resin junction in a water supply system, it is the thread roots that were attacked first, causing a brittle crack to grow. Discolouration on the fracture surface was caused by deposition of carbonates from the hard water
supply, so the joint had been in a critical state for many months. The problems in the US also occurred to polybutylene
pipework, and led to the material being removed from that market, although it is still used elsewhere in the world.
s can be biologically degraded by microorganism
s to give lower molecular weight molecules. To degrade properly biodegradable polymers need to be treated like compost
and not just left in a landfill site where degradation is very difficult due to the lack of oxygen and moisture.
light stabilisers (HALS) stabilise against weathering by scavenging free radicals that are produced by photo-oxidation of the polymer matrix. UV-absorbers
stabilises against weathering by absorbing ultraviolet light and converting it into heat. Antioxidant
s stabilize the polymer by terminating the chain reaction due to the absorption of UV light from sunlight. The chain reaction initiated by photo-oxidation leads to cessation of crosslinking of the polymers and degradation the property of polymers.
Tensile strength
Ultimate tensile strength , often shortened to tensile strength or ultimate strength, is the maximum stress that a material can withstand while being stretched or pulled before necking, which is when the specimen's cross-section starts to significantly contract...
, colour, shape, etc.—of a polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
or polymer-based product under the influence of one or more environmental factors such as heat
Heat
In physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between...
, light
Light
Light or visible light is electromagnetic radiation that is visible to the human eye, and is responsible for the sense of sight. Visible light has wavelength in a range from about 380 nanometres to about 740 nm, with a frequency range of about 405 THz to 790 THz...
or chemicals such as acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
s, alkali
Alkali
In chemistry, an alkali is a basic, ionic salt of an alkali metal or alkaline earth metal element. Some authors also define an alkali as a base that dissolves in water. A solution of a soluble base has a pH greater than 7. The adjective alkaline is commonly used in English as a synonym for base,...
s and some salts. These changes are usually undesirable, such as cracking and chemical disintegration of products or, more rarely, desirable, as in biodegradation
Biodegradation
Biodegradation or biotic degradation or biotic decomposition is the chemical dissolution of materials by bacteria or other biological means...
, or deliberately lowering the molecular weight of a polymer for recycling
Recycling
Recycling is processing used materials into new products to prevent waste of potentially useful materials, reduce the consumption of fresh raw materials, reduce energy usage, reduce air pollution and water pollution by reducing the need for "conventional" waste disposal, and lower greenhouse...
. The changes in properties are often termed "aging".
In a finished product such a change is to be prevented or delayed. Degradation can be useful for recycling
Recycling
Recycling is processing used materials into new products to prevent waste of potentially useful materials, reduce the consumption of fresh raw materials, reduce energy usage, reduce air pollution and water pollution by reducing the need for "conventional" waste disposal, and lower greenhouse...
/reusing the polymer waste to prevent or reduce environmental pollution
Pollution
Pollution is the introduction of contaminants into a natural environment that causes instability, disorder, harm or discomfort to the ecosystem i.e. physical systems or living organisms. Pollution can take the form of chemical substances or energy, such as noise, heat or light...
. Degradation can also be induced deliberately to assist structure determination.
Polymeric molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
s are very large (on the molecular scale), and their unique and useful properties are mainly a result of their size. Any loss in chain length lowers tensile strength and is a primary cause of premature cracking.
Commodity polymers
Today there are primarily seven commodity polymers in use: polyethylenePolyethylene
Polyethylene or polythene is the most widely used plastic, with an annual production of approximately 80 million metric tons...
, polypropylene
Polypropylene
Polypropylene , also known as polypropene, is a thermoplastic polymer used in a wide variety of applications including packaging, textiles , stationery, plastic parts and reusable containers of various types, laboratory equipment, loudspeakers, automotive components, and polymer banknotes...
, polyvinyl chloride
Polyvinyl chloride
Polyvinyl chloride, commonly abbreviated PVC, is a thermoplastic polymer. It is a vinyl polymer constructed of repeating vinyl groups having one hydrogen replaced by chloride. Polyvinyl chloride is the third most widely produced plastic, after polyethylene and polypropylene. PVC is widely used in...
, polyethylene terephthalate
Polyethylene terephthalate
Polyethylene terephthalate , commonly abbreviated PET, PETE, or the obsolete PETP or PET-P, is a thermoplastic polymer resin of the polyester family and is used in synthetic fibers; beverage, food and other liquid containers; thermoforming applications; and engineering resins often in combination...
, polystyrene
Polystyrene
Polystyrene ) also known as Thermocole, abbreviated following ISO Standard PS, is an aromatic polymer made from the monomer styrene, a liquid hydrocarbon that is manufactured from petroleum by the chemical industry...
, polycarbonate
Polycarbonate
PolycarbonatePhysical PropertiesDensity 1.20–1.22 g/cm3Abbe number 34.0Refractive index 1.584–1.586FlammabilityV0-V2Limiting oxygen index25–27%Water absorption – Equilibrium0.16–0.35%Water absorption – over 24 hours0.1%...
, and poly(methyl methacrylate) (Plexiglass). These make up nearly 98% of all polymers and plastics encountered in daily life. Each of these polymers has its own characteristic modes of degradation and resistances to heat, light and chemicals. Polyethylene, polypropylene, and poly(methyl methacrylate) are sensitive to oxidation and UV radiation, while PVC may discolour at high temperatures due to loss of hydrogen chloride
Hydrogen chloride
The compound hydrogen chloride has the formula HCl. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric humidity. Hydrogen chloride gas and hydrochloric acid are important in technology and industry...
gas, and become very brittle. PET is sensitive to hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
and attack by strong acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
s, while polycarbonate depolymerizes rapidly when exposed to strong alkali
Alkali
In chemistry, an alkali is a basic, ionic salt of an alkali metal or alkaline earth metal element. Some authors also define an alkali as a base that dissolves in water. A solution of a soluble base has a pH greater than 7. The adjective alkaline is commonly used in English as a synonym for base,...
s.
For example, polyethylene usually degrades by random scission - that is by a random breakage of the linkages (bonds) that hold the atoms of the polymer together. When this polymer is heated above 450 Celsius
Celsius
Celsius is a scale and unit of measurement for temperature. It is named after the Swedish astronomer Anders Celsius , who developed a similar temperature scale two years before his death...
it becomes a complex mixture of molecules of various sizes that resemble gasoline. Other polymers - like polyalphamethylstyrene - undergo 'unspecific' chain scission with breakage occurring only at the ends; they literally unzip or depolymerize to become the constituent monomers.


Photoinduced degradation
Most polymers can be degraded by photolysis to give lower molecular weight molecules. Electromagnetic waves with the energy of visible light or higher, such as ultraviolet light, X-rayX-ray
X-radiation is a form of electromagnetic radiation. X-rays have a wavelength in the range of 0.01 to 10 nanometers, corresponding to frequencies in the range 30 petahertz to 30 exahertz and energies in the range 120 eV to 120 keV. They are shorter in wavelength than UV rays and longer than gamma...
s and gamma ray
Gamma ray
Gamma radiation, also known as gamma rays or hyphenated as gamma-rays and denoted as γ, is electromagnetic radiation of high frequency . Gamma rays are usually naturally produced on Earth by decay of high energy states in atomic nuclei...
s are usually involved in such reactions.
Thermal degradation
Chain-growth polymers like poly(methyl methacrylate) can be degraded by thermolysis at high temperatures to give monomers, oils, gases and water. The degradation takes place by:| Thermolysis type | Added material | Temperature | Pressure | Final product |
|---|---|---|---|---|
| Pyrolysis Pyrolysis Pyrolysis is a thermochemical decomposition of organic material at elevated temperatures without the participation of oxygen. It involves the simultaneous change of chemical composition and physical phase, and is irreversible... |
Around 500°C | Reduced pressure | ||
| Hydrogenation Hydrogenation Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically... |
Dihydrogen | Around 450°C | Around 200 bars | |
| Gasification Gasification Gasification is a process that converts organic or fossil based carbonaceous materials into carbon monoxide, hydrogen, carbon dioxide and methane. This is achieved by reacting the material at high temperatures , without combustion, with a controlled amount of oxygen and/or steam... |
Dioxygen and/or water Water Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a... |
Under pressure | Carbon monoxide Carbon monoxide Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal... , Carbon dioxide Carbon dioxide Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom... and hydrogen Hydrogen Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly... |
Solvolysis
Step-growth polymers like polyesterPolyester
Polyester is a category of polymers which contain the ester functional group in their main chain. Although there are many polyesters, the term "polyester" as a specific material most commonly refers to polyethylene terephthalate...
s, polyamide
Polyamide
A polyamide is a polymer containing monomers of amides joined by peptide bonds. They can occur both naturally and artificially, examples being proteins, such as wool and silk, and can be made artificially through step-growth polymerization or solid-phase synthesis, examples being nylons, aramids,...
s and polycarbonate
Polycarbonate
PolycarbonatePhysical PropertiesDensity 1.20–1.22 g/cm3Abbe number 34.0Refractive index 1.584–1.586FlammabilityV0-V2Limiting oxygen index25–27%Water absorption – Equilibrium0.16–0.35%Water absorption – over 24 hours0.1%...
s can be degraded by solvolysis
Solvolysis
Solvolysis is a special type of nucleophilic substitution or elimination where the nucleophile is a solvent molecule. For certain nucleophiles, there are specific terms for the type of solvolysis reaction...
and mainly hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
to give lower molecular weight molecules. The hydrolysis takes place in the presence of water containing an acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
or a base
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
as catalyst.
Polyamide
Nylon
Nylon is a generic designation for a family of synthetic polymers known generically as polyamides, first produced on February 28, 1935, by Wallace Carothers at DuPont's research facility at the DuPont Experimental Station...
is sensitive to degradation by acids and polyamide mouldings will crack when attacked by strong acids. For example, the fracture surface of a fuel connector showed the progressive growth of the crack from acid attack (Ch) to the final cusp (C) of polymer. The problem is known as stress corrosion cracking
Stress corrosion cracking
Stress corrosion cracking is the unexpected sudden failure of normally ductile metals subjected to a tensile stress in a corrosive environment, especially at elevated temperature in the case of metals. SCC is highly chemically specific in that certain alloys are likely to undergo SCC only when...
, and in this case was caused by hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
of the polymer. It was the reverse reaction of the synthesis of the polymer:
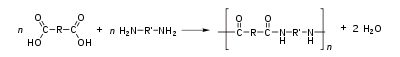
Ozonolysis

Ozone
Ozone , or trioxygen, is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic allotrope...
attack. Tiny traces of the gas in the air will attack double bonds in rubber chains, with Natural rubber, polybutadiene
Polybutadiene
Polybutadiene is a synthetic rubber that is a polymer formed from the polymerization process of the monomer 1,3-butadiene.It has a high resistance to wear and is used especially in the manufacture of tires, which consumes about 70% of the production...
, Styrene-butadiene
Styrene-butadiene
Styrene-Butadiene or Styrene-Butadiene-Rubber is a synthetic rubber copolymer consisting of styrene and butadiene. It has good abrasion resistance and good aging stability when protected by additives, and is widely used in car tires, where it may be blended with natural rubber...
rubber and NBR being most sensitive to degradation. Ozone cracks form in products under tension, but the critical strain is very small. The cracks are always oriented at right angles to the strain axis, so will form around the circumference in a rubber tube bent over. Such cracks are dangerous when they occur in fuel pipes because the cracks will grow from the outside exposed surfaces into the bore of the pipe, and fuel leakage and fire may follow. The problem of ozone cracking
Ozone cracking
Cracks can be formed in many different elastomers by ozone attack, and the characteristic form of attack of vulnerable rubbers is known as ozone cracking...
can be prevented by adding anti-ozonants to the rubber before vulcanization
Vulcanization
Vulcanization or vulcanisation is a chemical process for converting rubber or related polymers into more durable materials via the addition of sulfur or other equivalent "curatives." These additives modify the polymer by forming crosslinks between individual polymer chains. Vulcanized material is...
. Ozone cracks were commonly seen in automobile tire
Tire
A tire or tyre is a ring-shaped covering that fits around a wheel rim to protect it and enable better vehicle performance by providing a flexible cushion that absorbs shock while keeping the wheel in close contact with the ground...
sidewalls, but are now seen rarely thanks to these additives. On the other hand, the problem does recur in unprotected products such as rubber tubing and seals.
Oxidation

Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
, especially at elevated temperatures encountered during processing to shape. Many process methods such as extrusion
Extrusion
Extrusion is a process used to create objects of a fixed cross-sectional profile. A material is pushed or drawn through a die of the desired cross-section...
and injection moulding
Injection moulding
Injection molding is a manufacturing process for producing parts from both thermoplastic and thermosetting plastic materials. Material is fed into a heated barrel, mixed, and forced into a mold cavity where it cools and hardens to the configuration of the cavity...
involve pumping molten polymer into tools, and the high temperatures needed for melting may result in oxidation unless precautions are taken. For example, a forearm crutch
Crutch
Crutches are mobility aids used to counter a mobility impairment or an injury that limits walking ability.- Types :There are several different types of crutches:...
suddenly snapped and the user was severely injured in the resulting fall. The crutch had fractured across a polypropylene
Polypropylene
Polypropylene , also known as polypropene, is a thermoplastic polymer used in a wide variety of applications including packaging, textiles , stationery, plastic parts and reusable containers of various types, laboratory equipment, loudspeakers, automotive components, and polymer banknotes...
insert within the aluminium tube of the device, and infra-red spectroscopy of the material showed that it had oxidised, possible as a result of poor moulding.
Oxidation is usually relatively easy to detect owing to the strong absorption by the carbonyl group in the spectrum of polyolefins. Polypropylene
Polypropylene
Polypropylene , also known as polypropene, is a thermoplastic polymer used in a wide variety of applications including packaging, textiles , stationery, plastic parts and reusable containers of various types, laboratory equipment, loudspeakers, automotive components, and polymer banknotes...
has a relatively simple spectrum with few peaks at the carbonyl position (like polyethylene
Polyethylene
Polyethylene or polythene is the most widely used plastic, with an annual production of approximately 80 million metric tons...
). Oxidation tends to start at tertiary carbon atoms because the free radicals formed here are more stable and longer lasting, making them more susceptible to attack by oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
. The carbonyl group can be further oxidised to break the chain, this weakens the material by lowering its molecular weight, and cracks start to grow in the regions affected.
Galvanic action
Polymer degradation by galvanic action was first described in the technical literature in 1990. This was the discovery that "plastics can corrode", i.e. polymer degradation may occur through galvanic action similar to that of metals under certain conditions. Normally, when two dissimilar metals such as copperCopper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
(Cu) and iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
(Fe) are put into contact and then immersed in salt water, the iron will undergo corrosion
Corrosion
Corrosion is the disintegration of an engineered material into its constituent atoms due to chemical reactions with its surroundings. In the most common use of the word, this means electrochemical oxidation of metals in reaction with an oxidant such as oxygen...
, or rust. This is called a galvanic circuit where the copper is the noble metal
Noble metal
Noble metals are metals that are resistant to corrosion and oxidation in moist air, unlike most base metals. They tend to be precious, often due to their rarity in the Earth's crust...
and the iron is the active metal, i.e., the copper is the cathode
Cathode
A cathode is an electrode through which electric current flows out of a polarized electrical device. Mnemonic: CCD .Cathode polarity is not always negative...
or positive (+) electrode
Electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit...
and the iron is the anode
Anode
An anode is an electrode through which electric current flows into a polarized electrical device. Mnemonic: ACID ....
, or negative (-) electrode
Electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit...
. A battery
Battery (electricity)
An electrical battery is one or more electrochemical cells that convert stored chemical energy into electrical energy. Since the invention of the first battery in 1800 by Alessandro Volta and especially since the technically improved Daniell cell in 1836, batteries have become a common power...
is formed. It follows that plastics are made stronger by impregnating them with thin carbon fibers only a few micrometers in diameter known as carbon fiber
Carbon fiber
Carbon fiber, alternatively graphite fiber, carbon graphite or CF, is a material consisting of fibers about 5–10 μm in diameter and composed mostly of carbon atoms. The carbon atoms are bonded together in crystals that are more or less aligned parallel to the long axis of the fiber...
reinforced polymers (CFRP). This is to produce materials that are high strength and resistant to high temperatures. The carbon fibers act as a noble metal similar to gold (Au) or platinum (Pt). When put into contact with a more active metal, for example with aluminum (Al) in salt water the aluminum corrodes. However in early 1990, it was reported that imide-linked resins in CFRP composite
Composite material
Composite materials, often shortened to composites or called composition materials, are engineered or naturally occurring materials made from two or more constituent materials with significantly different physical or chemical properties which remain separate and distinct at the macroscopic or...
s degrade when bare composite is coupled with an active metal in salt water environments. This is because corrosion not only occurs at the aluminum anode
Anode
An anode is an electrode through which electric current flows into a polarized electrical device. Mnemonic: ACID ....
, but also at the carbon fiber
Carbon fiber
Carbon fiber, alternatively graphite fiber, carbon graphite or CF, is a material consisting of fibers about 5–10 μm in diameter and composed mostly of carbon atoms. The carbon atoms are bonded together in crystals that are more or less aligned parallel to the long axis of the fiber...
cathode
Cathode
A cathode is an electrode through which electric current flows out of a polarized electrical device. Mnemonic: CCD .Cathode polarity is not always negative...
in the form of a very strong base with a pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
of about 13. This strong base reacts with the polymer chain structure degrading the polymer. Polymers affected include bismaleimides (BMI), condensation polyimide
Polyimide
Polyimide is a polymer of imide monomers. The structure of imide is as shown. Polyimides have been in mass production since 1955...
s, triazine
Triazine
A triazine is one of three organic chemicals, isomeric with each other, whose molecular formula is 333 and whose empirical formula is CHN.- Structure :...
s, and blends thereof. Degradation occurs in the form of dissolved resin and loose fibers. The hydroxyl ions generated at the graphite cathode
Cathode
A cathode is an electrode through which electric current flows out of a polarized electrical device. Mnemonic: CCD .Cathode polarity is not always negative...
attack the O-C-N bond in the polyimide structure. Standard corrosion protection procedures were found to prevent polymer degradation under most conditions.
Chlorine-induced cracking

Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
, which will attack susceptible polymers such as acetal resin and polybutylene
Polybutylene
Polybutylene is a polyolefin or saturated polymer with the chemical formula n. It should not be confused with polybutene, a low molecular weight oligomer with a different repeat unit....
pipework. There have been many examples of such pipes and acetal fittings failing in properties in the US as a result of chlorine-induced cracking. In essence, the gas attacks sensitive parts of the chain molecules (especially secondary, tertiary, or allylic carbon atoms), oxidizing the chains and ultimately causing chain cleavage. The root cause is traces of chlorine in the water supply, added for its anti-bacterial action, attack occurring even at parts per million traces of the dissolved gas. The chlorine attacks weak parts of a product, and in the case of an acetal resin junction in a water supply system, it is the thread roots that were attacked first, causing a brittle crack to grow. Discolouration on the fracture surface was caused by deposition of carbonates from the hard water
Hard water
Hard water is water that has high mineral content . Hard water has high concentrations of Ca2+ and Mg2+ ions. Hard water is generally not harmful to one's health but can pose serious problems in industrial settings, where water hardness is monitored to avoid costly breakdowns in boilers, cooling...
supply, so the joint had been in a critical state for many months. The problems in the US also occurred to polybutylene
Polybutylene
Polybutylene is a polyolefin or saturated polymer with the chemical formula n. It should not be confused with polybutene, a low molecular weight oligomer with a different repeat unit....
pipework, and led to the material being removed from that market, although it is still used elsewhere in the world.
Biological degradation
Biodegradable plasticBiodegradable plastic
Biodegradable plastics are plastics that will decompose in natural aerobic and anaerobic environments. Biodegradation of plastics can be achieved by enabling microorganisms in the environment to metabolize the molecular structure of plastic films to produce an inert humus-like material that is...
s can be biologically degraded by microorganism
Microorganism
A microorganism or microbe is a microscopic organism that comprises either a single cell , cell clusters, or no cell at all...
s to give lower molecular weight molecules. To degrade properly biodegradable polymers need to be treated like compost
Compost
Compost is organic matter that has been decomposed and recycled as a fertilizer and soil amendment. Compost is a key ingredient in organic farming. At its most essential, the process of composting requires simply piling up waste outdoors and waiting for the materials to break down from anywhere...
and not just left in a landfill site where degradation is very difficult due to the lack of oxygen and moisture.
Stabilisers
Hindered amineHindered amine
Hindered amines are chemical compounds containing an amine functional group surrounded by a crowded steric environment. They have uses such as gas scrubbing, as stabilizers against light-induced polymer degradation, and as reagents for organic synthesis....
light stabilisers (HALS) stabilise against weathering by scavenging free radicals that are produced by photo-oxidation of the polymer matrix. UV-absorbers
UV Stabilizers in plastics
UV light stabilizers are used frequently in plastics, including cosmetics and films. The primary function is to protect the substance from the long-term degradation effects from light, most frequently ultraviolet light...
stabilises against weathering by absorbing ultraviolet light and converting it into heat. Antioxidant
Antioxidant
An antioxidant is a molecule capable of inhibiting the oxidation of other molecules. Oxidation is a chemical reaction that transfers electrons or hydrogen from a substance to an oxidizing agent. Oxidation reactions can produce free radicals. In turn, these radicals can start chain reactions. When...
s stabilize the polymer by terminating the chain reaction due to the absorption of UV light from sunlight. The chain reaction initiated by photo-oxidation leads to cessation of crosslinking of the polymers and degradation the property of polymers.
See also
- Applied spectroscopyApplied spectroscopyApplied spectroscopy is the application of various spectroscopic methods for detection and identification of different elements/compounds in solving problems in the fields of forensics, medicine, oil industry, atmospheric chemistry, pharmacology, etc....
- Forensic engineeringForensic engineeringForensic engineering is the investigation of materials, products, structures or components that fail or do not operate or function as intended, causing personal injury or damage to property. The consequences of failure are dealt with by the law of product liability. The field also deals with...
- Forensic materials engineeringForensic materials engineeringA branch of Forensic engineering, the subject focuses on the material evidence from crime or accident scenes, seeking defects in those materials which might explain why an accident occurred, or the source of a specific material to identify a criminal...
- Forensic polymer engineeringForensic polymer engineeringThe study of failure in polymeric products is called forensic polymer engineering. The topic includes the fracture of plastic products, or any other reason why such a product fails in service, or fails to meet its specification...
- Environmental stress fractureEnvironmental stress fractureIn materials science, environmental stress fracture or environment assisted fracture is the generic name given to premature failure under the influence of tensile stresses and harmful environments of materials such as metals and alloys, composites, plastics and ceramics.Metals and alloys exhibit...
- Polymer engineeringPolymer engineeringPolymer engineering is generally an engineering field that designs, analyses, and/or modifies polymer materials. Polymer engineering covers aspects of petrochemical industry, polymerization, structure and characterization of polymers, properties of polymers, compounding and processing of polymers...
- PolymerPolymerA polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
- Stress corrosion crackingStress corrosion crackingStress corrosion cracking is the unexpected sudden failure of normally ductile metals subjected to a tensile stress in a corrosive environment, especially at elevated temperature in the case of metals. SCC is highly chemically specific in that certain alloys are likely to undergo SCC only when...
- Environmental stress crackingEnvironmental stress crackingEnvironmental Stress Cracking is one of the most common causes of unexpected brittle failure of thermoplastic polymers known at present. Environmental stress cracking may account for around 15-30% of all plastic component failures in service.ESC and polymer resistance to ESC have been studied...
- Weather testing of polymersWeather testing of polymersWeather testing of polymers is the controlled polymer degradation and polymer coating degradation under lab or natural conditions.Just like erosion of rocks, natural phenomena can cause degradation in polymer systems. The elements of most concern to polymers are Ultraviolet radiation, moisture and...

