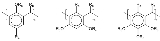
Calixarene
Encyclopedia
A calixarene is a macrocycle
or cyclic
oligomer
based on a hydroxyalkylation product of a phenol
and an aldehyde
. The word calixarene is derived from calix or chalice
because this type of molecule resembles a vase and from the word arene
that refers to the aromatic building block. Calixarenes have hydrophobic cavities that can hold smaller molecules or ions and belong to the class of cavitand
s known in host-guest chemistry
. Calixarene nomenclature
is straightforward and involves counting the number of repeating units in the ring and include it in the name. A calix[4]arene has 4 units in the ring and a calix[6]arene has 6. A substituent
in the meso position Rb is added to the name with a prefix C- as in C-methylcalix[6]arene.
, resorcinol
or pyrogallol
, For phenol, the aldehyde most often used is simply formaldehyde
, while larger aldehydes (acetaldehyde
, or larger) are generally required in condensation reactions with resorcinol and pyrogallol. The chemical reaction
ranks under electrophilic aromatic substitution
s followed by an elimination
of water and then a second aromatic substitution. The reaction is acid
catalysed or base
catalysed
. Calixarenes are difficult to produce because it is all too easy to end up with complex mixtures of linear and cyclic oligomers with different numbers of repeating units. With finely tuned starting materials and reaction conditions synthesis can also be surprisingly easy. In 2005, research produced a pyrogallol[4]arene by simply mixing a solvent-free dispersion of isovaleraldehyde with pyrogallol and a catalytic amount of p-toluenesulfonic acid
in a mortar and pestle
. Calixarenes as parent compounds are sparingly soluble and are high melting crystalline solids.
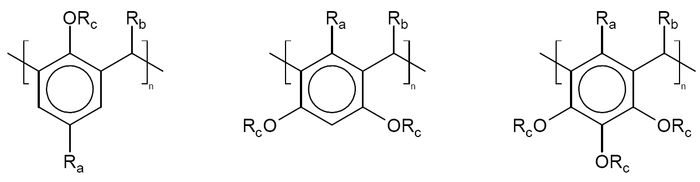
is around 10 cubic nanometers. Calixarenes are characterised by a wide upper rim and a narrow lower rim and a central annulus. With phenol as a starting material the 4 hydroxyl
groups are intrannular on the lower rim. In a resorcin[4]arene 8 hydroxyl groups are placed extraannular on the upper ring. Calixarenes exist in different chemical conformations because rotation around the methylene bridge is not difficult. In calix[4]arene 4 up-down conformations exist: cone (point group
C2v,C4v), partial cone Cs, 1,2 alternate C2h and 1,3 alternate D2d. The 4 hydroxyl groups interact by hydrogen bond
ing and stabilize the cone conformation. This conformation is in dymamic equilibrium with the other conformations. Conformations can be locked in place with proper substituents replacing the hydroxyl groups which increase the rotational barrier. Alternatively placing a bulky substituent on the upper rim also locks a conformation. The calixarene based on p-tert-butyl phenol is also a cone.http://www.iupac.org/publications/pac/1993/pdf/6503x0387.pdf Calixarenes are structurally related to the pillararene
s.
pioneered the chemistry of calixarenes although he was unable to determine its structure and did not realise its potential (he was pursuing dyes). In 1872 he mixed benzaldehyde
with pyrogallol
and a strong acid
and noted a red-brown resin
with a marked viscosity
increase. He also used resorcinol
and formaldehyde
which he had to prepare from iodoform
himself because a commercial grade of formaldehyde at that time had not been realised yet. In 1894 the Lederer-Manasse hydroxyalkylation was invented as a synthetic tool for the preparation of hydroxylmethyl phenols, bringing calixarenes one step closer. In 1902 Leo Baekeland
made phenol formaldehyde resin
s a commercial success under the trade name Bakelite. In these resins phenol and formaldehyde are exhaustively condensed with each other to form heavily cross-link
ed polymers. The first attempt to control the reaction was made by Alois Zinke and Erich Ziegler in 1942. They employed para substituted phenols which inhibits crosslinking and should result in a linear polymer with formaldehyde. So in 1944 p-tert-butyl phenol with formaldehyde and sodium hydroxide in linseed oil
as a solvent produced for the first time a crystalline solid with a high melting point rather than a resin. In the same year another duo by the names of Niederl and Vogel did something similar with a para substituted resorcinol
and they were the first to postulate a cyclic tetramer. In these days structure elucidation was limited to determination of molar mass
by freezing-point depression
and functional group
analysis.
John Cornforth
was in 1955 the first to realize the potential of calixarenes as a basket analogue to enzyme
s and repeated the work done by Zinke. He obtained a mixture of products and elicited the services of Dorothy Crowfoot Hodgkin
for structure elucidation by X-ray crystallography
but with limited success. First commercial success came to calixarenes in the nineteen fifties when the company Petrolite started a range of calixarene products as demulsifier
s used in the oil industry.
The word calixarene was coined by C. David Gutschein 1975 who was also interested in this type of compound as biomimetic, since the molecule resembled the calyx krater vases of ancient Greece. It was by then established that unmodified calixarenes exhibit extensive conformational mobility so that the basket was not much of a basket after all. Donald J. Cram
fixed this shortcoming by inventing a way of immobilizing calixarenes. He was able to freeze in a conformation by so called lower rim functionalization, replacing the hydroxyl
groups by larger substituent
s. The acetate
calixarene fixates the molecule as a partial cone, whereas the carbonate ester
yields the full cone.
ionophore
s and are applied as such in chemical sensor
s. With the right chemistry these molecules exhibit great selectivity towards other cations. Calixarenes are used in commercial applications as sodium selective electrode
s for the measurement of sodium levels in blood. Calixarenes also form complexes with cadmium
, lead
, lanthanide
s and actinide
s. Calix[5]arene and the C70 fullerene
in p-xylene
form a ball-and-socket supramolecular complex. Calixarenes also form exo-calix ammonium salts with aliphatic amines such as piperidine
.
of resorcinarenes and pyrogallolarenes lead to larger supramolecular assemblies. Both in the crystalline state and in solution, they are known to form hexamers that are akin to certain Archimedean solid
s with an internal volume of around one cubic nanometer (nanocapsules). (Isobutylpyrogallol[4]arene)6 is held together by 48 intermolecular hydrogen bonds. The remaining 24 hydrogen bonds are intramolecular
. The cavity is filled by a number of solvent molecules.
stationary phases. In addition, in nanotechnology
calixarenes are used as negative resist for high-resolution electron beam lithography
http://dx.doi.org/10.1063/1.115958.
A tetrathia[4]arene is found to mimic aquaporin
proteins. This calixarene adopts a 1,3-alternate conformation (methoxy groups populate the lower ring) and water is not contained in the basket but grabbed by two opposing tert-butyl groups on the outer rim in a pincer. The nonporous and hydrophobic crystals are soaked in water for 8 hours in which time the calixarene:water ratio nevertheless acquires the value of one.
Calixarenes are able to accelerate reactions taking place inside the concavity by a combination of local concentration effect and polar stabilization of the transition state
. An extended resorcin[4]arene cavitand
is found to accelerate the reaction rate
of a Menshutkin reaction
between quinuclidine
and butylbromide by a factor of 1600.
In heterocalixarenes the phenolic units are replaced by heterocycles, for instance by furan
s in calix[n]furanes and by pyridine
s in calix[n]pyridines. Calixarenes have been used as the macrocycle
portion of a rotaxane
and two calixarene molecules covalently joined together by the lower rims form carcerand
s.
Calix[4]arene was used as scaffold to assemble a construct bearing four Tn-antigen unit, at upper rim, and the immunoadjuvant P3CS, at lower rim. The construct showed a cluster effect in the production of Tn specific IgG antibodies in mice when compared to an analogous monovalent construct. This reveals perspectives for potential applications in
cancer immunotherapy.
. Recently, inherently chiral calixarenes have been synthesised in good yields by asymmetric ortholithiation using a chiral oxazoline directing group. This removes the need for resolution techniques.
Macrocycle
A macrocycle is, as defined by IUPAC, "a cyclic macromolecule or a macromolecular cyclic portion of a molecule." In the chemical literature, organic chemists may consider any molecule containing a ring of nine or more atoms to be a macrocycle...
or cyclic
Cyclic compound
In chemistry, a cyclic compound is a compound in which a series of atoms is connected to form a loop or ring.While the vast majority of cyclic compounds are organic, a few inorganic substances form cyclic compounds as well, including sulfur, silanes, phosphanes, phosphoric acid, and triboric acid. ...
oligomer
Oligomer
In chemistry, an oligomer is a molecule that consists of a few monomer units , in contrast to a polymer that, at least in principle, consists of an unlimited number of monomers. Dimers, trimers, and tetramers are oligomers. Many oils are oligomeric, such as liquid paraffin...
based on a hydroxyalkylation product of a phenol
Phenol
Phenol, also known as carbolic acid, phenic acid, is an organic compound with the chemical formula C6H5OH. It is a white crystalline solid. The molecule consists of a phenyl , bonded to a hydroxyl group. It is produced on a large scale as a precursor to many materials and useful compounds...
and an aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
. The word calixarene is derived from calix or chalice
Chalice (cup)
A chalice is a goblet or footed cup intended to hold a drink. In general religious terms, it is intended for drinking during a ceremony.-Christian:...
because this type of molecule resembles a vase and from the word arene
Arene
Arene or Arênê or Arène may refer to:*an aromatic hydrocarbon*Arene , a genus of marine snails in the family Areneidae*Arene , the wife of Aphareus and mother of Idas and Lynceus in Greek mythology...
that refers to the aromatic building block. Calixarenes have hydrophobic cavities that can hold smaller molecules or ions and belong to the class of cavitand
Cavitand
A cavitand is a container shaped molecule. The cavity of the cavitand allows it to engage in host-guest chemistry with guest molecules of a complementary shape and size. Examples include cyclodextrins, calixarenes, pillararenes and cucurbiturils....
s known in host-guest chemistry
Host-guest chemistry
In supramolecular chemistry, host-guest chemistry describes complexes that are composed of two or more molecules or ions that are held together in unique structural relationships by forces other than those of full covalent bonds. Host-guest chemistry encompasses the idea of molecular recognition...
. Calixarene nomenclature
IUPAC nomenclature
A chemical nomenclature is a set of rules to generate systematic names for chemical compounds. The nomenclature used most frequently worldwide is the one created and developed by the International Union of Pure and Applied Chemistry ....
is straightforward and involves counting the number of repeating units in the ring and include it in the name. A calix[4]arene has 4 units in the ring and a calix[6]arene has 6. A substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
in the meso position Rb is added to the name with a prefix C- as in C-methylcalix[6]arene.
Synthesis
The aromatic components are derived from phenolPhenol
Phenol, also known as carbolic acid, phenic acid, is an organic compound with the chemical formula C6H5OH. It is a white crystalline solid. The molecule consists of a phenyl , bonded to a hydroxyl group. It is produced on a large scale as a precursor to many materials and useful compounds...
, resorcinol
Resorcinol
Resorcinol is a dihydroxy benzene. It is the 1,3-isomer of benzenediol with the formula C6H42.-Nomenclature:Benzene-1,3-diol is the name recommended by the International Union of Pure and Applied Chemistry in its 1993 Recommendations for the Nomenclature of Organic Chemistry.-Production:It is...
or pyrogallol
Pyrogallol
Pyrogallol or benzene-1,2,3-triol is a benzenetriol. It is a white crystalline powder and a powerful reducing agent. It was first prepared by Scheele 1786 by heating gallic acid. An alternate preparation is heating para-chlorophenoldisulphonic acid with potassium hydroxide.When in alkaline...
, For phenol, the aldehyde most often used is simply formaldehyde
Formaldehyde
Formaldehyde is an organic compound with the formula CH2O. It is the simplest aldehyde, hence its systematic name methanal.Formaldehyde is a colorless gas with a characteristic pungent odor. It is an important precursor to many other chemical compounds, especially for polymers...
, while larger aldehydes (acetaldehyde
Acetaldehyde
Acetaldehyde is an organic chemical compound with the formula CH3CHO or MeCHO. It is one of the most important aldehydes, occurring widely in nature and being produced on a large scale industrially. Acetaldehyde occurs naturally in coffee, bread, and ripe fruit, and is produced by plants as part...
, or larger) are generally required in condensation reactions with resorcinol and pyrogallol. The chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
ranks under electrophilic aromatic substitution
Electrophilic aromatic substitution
Electrophilic aromatic substitution EAS is an organic reaction in which an atom, usually hydrogen, appended to an aromatic system is replaced by an electrophile...
s followed by an elimination
Elimination
- Science and medicine :*Elimination reaction, an organic reaction in which two functional groups split to form an organic product*Elimination, clearance of a drug or other foreign agent from the body...
of water and then a second aromatic substitution. The reaction is acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
catalysed or base
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
catalysed
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
. Calixarenes are difficult to produce because it is all too easy to end up with complex mixtures of linear and cyclic oligomers with different numbers of repeating units. With finely tuned starting materials and reaction conditions synthesis can also be surprisingly easy. In 2005, research produced a pyrogallol[4]arene by simply mixing a solvent-free dispersion of isovaleraldehyde with pyrogallol and a catalytic amount of p-toluenesulfonic acid
P-Toluenesulfonic acid
p-Toluenesulfonic acid or tosylic acid is an organic compound with the formula CH3C6H4SO3H. It is a white solid that is soluble in water, alcohols, and other polar organic solvents. The 4-CH3C6H4SO2- group is known as the Tosyl group and is often abbreviated as Ts or Tos...
in a mortar and pestle
Mortar and pestle
A mortar and pestle is a tool used to crush, grind, and mix solid substances . The pestle is a heavy bat-shaped object, the end of which is used for crushing and grinding. The mortar is a bowl, typically made of hard wood, ceramic or stone...
. Calixarenes as parent compounds are sparingly soluble and are high melting crystalline solids.

Structure
Calixarenes are characterised by a three-dimensional basket, cup or bucket shape. In calix[4]arenes the internal volumeVolume
Volume is the quantity of three-dimensional space enclosed by some closed boundary, for example, the space that a substance or shape occupies or contains....
is around 10 cubic nanometers. Calixarenes are characterised by a wide upper rim and a narrow lower rim and a central annulus. With phenol as a starting material the 4 hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
groups are intrannular on the lower rim. In a resorcin[4]arene 8 hydroxyl groups are placed extraannular on the upper ring. Calixarenes exist in different chemical conformations because rotation around the methylene bridge is not difficult. In calix[4]arene 4 up-down conformations exist: cone (point group
Point group
In geometry, a point group is a group of geometric symmetries that keep at least one point fixed. Point groups can exist in a Euclidean space with any dimension, and every point group in dimension d is a subgroup of the orthogonal group O...
C2v,C4v), partial cone Cs, 1,2 alternate C2h and 1,3 alternate D2d. The 4 hydroxyl groups interact by hydrogen bond
Hydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
ing and stabilize the cone conformation. This conformation is in dymamic equilibrium with the other conformations. Conformations can be locked in place with proper substituents replacing the hydroxyl groups which increase the rotational barrier. Alternatively placing a bulky substituent on the upper rim also locks a conformation. The calixarene based on p-tert-butyl phenol is also a cone.http://www.iupac.org/publications/pac/1993/pdf/6503x0387.pdf Calixarenes are structurally related to the pillararene
Pillararene
Pillararenes are macrocycles composed of hydroquinone units linked in the para position. They are structurally similar to the cucurbiturils and calixarenes that play an important part in host-guest chemistry. arene], the first pillarene, was synthesized by Tomoki Ogoshi at Kanazawa University....
s.
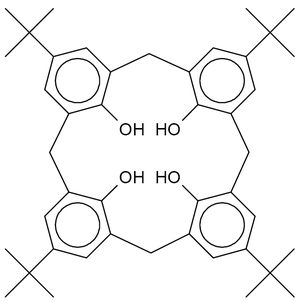 |
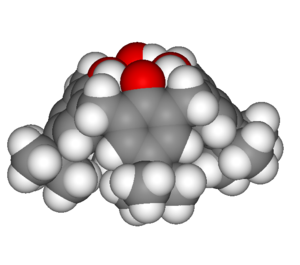 |
| Calix[4]arene with para-tert-butyl substituents | 3D representation of a cone conformation |
History
Adolf von BaeyerAdolf von Baeyer
Johann Friedrich Wilhelm Adolf von Baeyer was a German chemist who synthesized indigo, and was the 1905 recipient of the Nobel Prize in Chemistry. Born in Berlin, he initially studied mathematics and physics at Berlin University before moving to Heidelberg to study chemistry with Robert Bunsen...
pioneered the chemistry of calixarenes although he was unable to determine its structure and did not realise its potential (he was pursuing dyes). In 1872 he mixed benzaldehyde
Benzaldehyde
Benzaldehyde is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful. This colorless liquid has a characteristic pleasant almond-like odor...
with pyrogallol
Pyrogallol
Pyrogallol or benzene-1,2,3-triol is a benzenetriol. It is a white crystalline powder and a powerful reducing agent. It was first prepared by Scheele 1786 by heating gallic acid. An alternate preparation is heating para-chlorophenoldisulphonic acid with potassium hydroxide.When in alkaline...
and a strong acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
and noted a red-brown resin
Resin
Resin in the most specific use of the term is a hydrocarbon secretion of many plants, particularly coniferous trees. Resins are valued for their chemical properties and associated uses, such as the production of varnishes, adhesives, and food glazing agents; as an important source of raw materials...
with a marked viscosity
Viscosity
Viscosity is a measure of the resistance of a fluid which is being deformed by either shear or tensile stress. In everyday terms , viscosity is "thickness" or "internal friction". Thus, water is "thin", having a lower viscosity, while honey is "thick", having a higher viscosity...
increase. He also used resorcinol
Resorcinol
Resorcinol is a dihydroxy benzene. It is the 1,3-isomer of benzenediol with the formula C6H42.-Nomenclature:Benzene-1,3-diol is the name recommended by the International Union of Pure and Applied Chemistry in its 1993 Recommendations for the Nomenclature of Organic Chemistry.-Production:It is...
and formaldehyde
Formaldehyde
Formaldehyde is an organic compound with the formula CH2O. It is the simplest aldehyde, hence its systematic name methanal.Formaldehyde is a colorless gas with a characteristic pungent odor. It is an important precursor to many other chemical compounds, especially for polymers...
which he had to prepare from iodoform
Iodoform
Iodoform is the organoiodine compound with the formula CHI3. A pale yellow, crystalline, volatile substance, it has a penetrating odor and, analogous to chloroform, sweetish taste. It is occasionally used as a disinfectant...
himself because a commercial grade of formaldehyde at that time had not been realised yet. In 1894 the Lederer-Manasse hydroxyalkylation was invented as a synthetic tool for the preparation of hydroxylmethyl phenols, bringing calixarenes one step closer. In 1902 Leo Baekeland
Leo Baekeland
Leo Hendrik Baekeland was a Belgian chemist who invented Velox photographic paper and Bakelite , an inexpensive, nonflammable, versatile, and popular plastic, which marks the beginning of the modern plastics industry.-Career:Leo Baekeland was born in Sint-Martens-Latem near Ghent, Belgium,...
made phenol formaldehyde resin
Phenol formaldehyde resin
Phenol formaldehyde resins include synthetic thermosetting resins such as obtained by the reaction of phenols with formaldehyde. Sometimes the precursors include other aldehydes or other phenol. Phenolic resins are mainly used in the production of circuit boards...
s a commercial success under the trade name Bakelite. In these resins phenol and formaldehyde are exhaustively condensed with each other to form heavily cross-link
Cross-link
Cross-links are bonds that link one polymer chain to another. They can be covalent bonds or ionic bonds. "Polymer chains" can refer to synthetic polymers or natural polymers . When the term "cross-linking" is used in the synthetic polymer science field, it usually refers to the use of...
ed polymers. The first attempt to control the reaction was made by Alois Zinke and Erich Ziegler in 1942. They employed para substituted phenols which inhibits crosslinking and should result in a linear polymer with formaldehyde. So in 1944 p-tert-butyl phenol with formaldehyde and sodium hydroxide in linseed oil
Linseed oil
Linseed oil, also known as flaxseed oil, is a clear to yellowish oil obtained from the dried ripe seeds of the flax plant . The oil is obtained by cold pressing, sometimes followed by solvent extraction...
as a solvent produced for the first time a crystalline solid with a high melting point rather than a resin. In the same year another duo by the names of Niederl and Vogel did something similar with a para substituted resorcinol
Resorcinol
Resorcinol is a dihydroxy benzene. It is the 1,3-isomer of benzenediol with the formula C6H42.-Nomenclature:Benzene-1,3-diol is the name recommended by the International Union of Pure and Applied Chemistry in its 1993 Recommendations for the Nomenclature of Organic Chemistry.-Production:It is...
and they were the first to postulate a cyclic tetramer. In these days structure elucidation was limited to determination of molar mass
Molar mass
Molar mass, symbol M, is a physical property of a given substance , namely its mass per amount of substance. The base SI unit for mass is the kilogram and that for amount of substance is the mole. Thus, the derived unit for molar mass is kg/mol...
by freezing-point depression
Freezing-point depression
Freezing-point depression describes the phenomenon in which the freezing point of a liquid is depressed when another compound is added, meaning that a solution has a lower freezing point than a pure solvent. This happens whenever a non-volatile solute is added to a pure solvent, such as water...
and functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
analysis.
John Cornforth
John Cornforth
Sir John Warcup 'Kappa' Cornforth, AC, CBE, FRS , is an Australian scientist who won the Nobel Prize in Chemistry in 1975 for his work on the stereochemistry of enzyme-catalyzed reactions....
was in 1955 the first to realize the potential of calixarenes as a basket analogue to enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
s and repeated the work done by Zinke. He obtained a mixture of products and elicited the services of Dorothy Crowfoot Hodgkin
Dorothy Crowfoot Hodgkin
Dorothy Mary Hodgkin OM, FRS , née Crowfoot, was a British chemist, credited with the development of protein crystallography....
for structure elucidation by X-ray crystallography
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
but with limited success. First commercial success came to calixarenes in the nineteen fifties when the company Petrolite started a range of calixarene products as demulsifier
Demulsifier
Demulsifiers, or emulsion breakers, are a class of specialty chemicals used to separate emulsions . They are commonly used in the processing of crude oil, which is typically produced along with significant quantities of saline water. This water must be removed from the crude oil prior to refining...
s used in the oil industry.
The word calixarene was coined by C. David Gutschein 1975 who was also interested in this type of compound as biomimetic, since the molecule resembled the calyx krater vases of ancient Greece. It was by then established that unmodified calixarenes exhibit extensive conformational mobility so that the basket was not much of a basket after all. Donald J. Cram
Donald J. Cram
Donald James Cram was an American chemist who shared the 1987 Nobel Prize in Chemistry with Jean-Marie Lehn and Charles J...
fixed this shortcoming by inventing a way of immobilizing calixarenes. He was able to freeze in a conformation by so called lower rim functionalization, replacing the hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
groups by larger substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
s. The acetate
Acetate
An acetate is a derivative of acetic acid. This term includes salts and esters, as well as the anion found in solution. Most of the approximately 5 billion kilograms of acetic acid produced annually in industry are used in the production of acetates, which usually take the form of polymers. In...
calixarene fixates the molecule as a partial cone, whereas the carbonate ester
Carbonate ester
A carbonate ester is a functional group in organic chemistry consisting of a carbonyl group flanked by two alkoxy groups. The general structure of these carbonates is R1OOR2 and they are related to esters R1OR and ethers R1OR2 and also to the inorganic carbonates.Carbonate esters are used as...
yields the full cone.
Host guest interactions
Calixarenes are efficient sodiumSodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
ionophore
Ionophore
An ionophore is a lipid-soluble molecule usually synthesized by microorganisms to transport ions across the lipid bilayer of the cell membrane...
s and are applied as such in chemical sensor
Sensor
A sensor is a device that measures a physical quantity and converts it into a signal which can be read by an observer or by an instrument. For example, a mercury-in-glass thermometer converts the measured temperature into expansion and contraction of a liquid which can be read on a calibrated...
s. With the right chemistry these molecules exhibit great selectivity towards other cations. Calixarenes are used in commercial applications as sodium selective electrode
Electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit...
s for the measurement of sodium levels in blood. Calixarenes also form complexes with cadmium
Cadmium
Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, bluish-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Similar to zinc, it prefers oxidation state +2 in most of its compounds and similar to mercury it shows a low...
, lead
Lead
Lead is a main-group element in the carbon group with the symbol Pb and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed...
, lanthanide
Lanthanide
The lanthanide or lanthanoid series comprises the fifteen metallic chemical elements with atomic numbers 57 through 71, from lanthanum through lutetium...
s and actinide
Actinide
The actinide or actinoid series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium.The actinide series derives its name from the group 3 element actinium...
s. Calix[5]arene and the C70 fullerene
Fullerene
A fullerene is any molecule composed entirely of carbon, in the form of a hollow sphere, ellipsoid, or tube. Spherical fullerenes are also called buckyballs, and they resemble the balls used in association football. Cylindrical ones are called carbon nanotubes or buckytubes...
in p-xylene
P-Xylene
p-Xylene is an aromatic hydrocarbon, based on benzene with two methyl substituents. The “p” stands for para, identifying the location of the methyl groups as across from one another....
form a ball-and-socket supramolecular complex. Calixarenes also form exo-calix ammonium salts with aliphatic amines such as piperidine
Piperidine
Piperidine is an organic compound with the molecular formula 5NH. This heterocyclic amine consists of a six-membered ring containing five methylene units and one nitrogen atom...
.
Molecular self-assembly
Molecular self-assemblyMolecular self-assembly
Molecular self-assembly is the process by which molecules adopt a defined arrangement without guidance or management from an outside source. There are two types of self-assembly, intramolecular self-assembly and intermolecular self-assembly...
of resorcinarenes and pyrogallolarenes lead to larger supramolecular assemblies. Both in the crystalline state and in solution, they are known to form hexamers that are akin to certain Archimedean solid
Archimedean solid
In geometry an Archimedean solid is a highly symmetric, semi-regular convex polyhedron composed of two or more types of regular polygons meeting in identical vertices...
s with an internal volume of around one cubic nanometer (nanocapsules). (Isobutylpyrogallol[4]arene)6 is held together by 48 intermolecular hydrogen bonds. The remaining 24 hydrogen bonds are intramolecular
Intramolecular
Intramolecular in chemistry describes a process or characteristic limited within the structure of a single molecule, a property or phenomenon limited to the extent of a single molecule.- Examples :...
. The cavity is filled by a number of solvent molecules.
Applications
Calixarenes are applied in enzyme mimetics, ion sensitive electrodes or sensors, selective membranes, non-linear optics and in HPLCHigh-performance liquid chromatography
High-performance liquid chromatography , HPLC, is a chromatographic technique that can separate a mixture of compounds and is used in biochemistry and analytical chemistry to identify, quantify and purify the individual components of the mixture.HPLC typically utilizes different types of stationary...
stationary phases. In addition, in nanotechnology
Nanotechnology
Nanotechnology is the study of manipulating matter on an atomic and molecular scale. Generally, nanotechnology deals with developing materials, devices, or other structures possessing at least one dimension sized from 1 to 100 nanometres...
calixarenes are used as negative resist for high-resolution electron beam lithography
Electron beam lithography
Electron beam lithography is the practice of emitting a beam of electrons in a patterned fashion across a surface covered with a film , and of selectively removing either exposed or non-exposed regions of the resist...
http://dx.doi.org/10.1063/1.115958.
A tetrathia[4]arene is found to mimic aquaporin
Aquaporin
Aquaporins are proteins embedded in the cell membrane that regulate the flow of water.Aquaporins are integral membrane proteins from a larger family of major intrinsic proteins that form pores in the membrane of biological cells....
proteins. This calixarene adopts a 1,3-alternate conformation (methoxy groups populate the lower ring) and water is not contained in the basket but grabbed by two opposing tert-butyl groups on the outer rim in a pincer. The nonporous and hydrophobic crystals are soaked in water for 8 hours in which time the calixarene:water ratio nevertheless acquires the value of one.
Calixarenes are able to accelerate reactions taking place inside the concavity by a combination of local concentration effect and polar stabilization of the transition state
Transition state
The transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
. An extended resorcin[4]arene cavitand
Cavitand
A cavitand is a container shaped molecule. The cavity of the cavitand allows it to engage in host-guest chemistry with guest molecules of a complementary shape and size. Examples include cyclodextrins, calixarenes, pillararenes and cucurbiturils....
is found to accelerate the reaction rate
Reaction rate
The reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
of a Menshutkin reaction
Menshutkin reaction
The Menshutkin reaction in organic chemistry converts a tertiary amine to a quaternary ammonium salt by reaction with an alkyl halide:The reaction has been named after its discoverer, the Russian chemist Nikolai Menshutkin, who described the procedure in 1890...
between quinuclidine
Quinuclidine
Quinuclidine is an organic compound and a bicyclic amine and used as a catalyst and a chemical building block. It is a strong base with pKa of the conjugate acid of 11.0. This is due to greater availability of the nitrogen lone pair...
and butylbromide by a factor of 1600.
In heterocalixarenes the phenolic units are replaced by heterocycles, for instance by furan
Furan
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen. The class of compounds containing such rings are also referred to as furans....
s in calix[n]furanes and by pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
s in calix[n]pyridines. Calixarenes have been used as the macrocycle
Macrocycle
A macrocycle is, as defined by IUPAC, "a cyclic macromolecule or a macromolecular cyclic portion of a molecule." In the chemical literature, organic chemists may consider any molecule containing a ring of nine or more atoms to be a macrocycle...
portion of a rotaxane
Rotaxane
A rotaxane is a mechanically-interlocked molecular architecture consisting of a "dumbbell shaped molecule" which is threaded through a "macrocycle" . The name is derived from the Latin for wheel and axle...
and two calixarene molecules covalently joined together by the lower rims form carcerand
Carcerand
A carcerand is a host molecule that completely entraps its guest so that it will not escape even at high temperatures. This type of molecule was first described by Donald J. Cram in 1985 and is derived from the Latin carcer, or prison...
s.
Calix[4]arene was used as scaffold to assemble a construct bearing four Tn-antigen unit, at upper rim, and the immunoadjuvant P3CS, at lower rim. The construct showed a cluster effect in the production of Tn specific IgG antibodies in mice when compared to an analogous monovalent construct. This reveals perspectives for potential applications in
cancer immunotherapy.
Inherent chirality
Calix[4]arenes with XXYZ or WXYZ substitution patterns at the upper rim are inherently chiral and their enantiomers can be resolved by chiral column chromatographyChiral column chromatography
Chiral column chromatography is a variant of column chromatography in which the stationary phase contains a single enantiomer of a chiral compound rather than being achiral...
. Recently, inherently chiral calixarenes have been synthesised in good yields by asymmetric ortholithiation using a chiral oxazoline directing group. This removes the need for resolution techniques.

