
Fluorescence in the life sciences
Encyclopedia
Fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation of a different wavelength. It is a form of luminescence. In most cases, emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation...
.
Some protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s or small molecule
Small molecule
In the fields of pharmacology and biochemistry, a small molecule is a low molecular weight organic compound which is by definition not a polymer...
s in cells are naturally fluorescent, which is called intrinsic fluorescence or autofluorescence
Autofluorescence
Autofluorescence is the natural emission of light by biological entities such as mitochondria and lysosomes, and is used to distinguish the light originating from artificially added fluorescent markers...
(such as NADH
Nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide, abbreviated NAD, is a coenzyme found in all living cells. The compound is a dinucleotide, since it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide.In metabolism, NAD is involved...
, tryptophan
Tryptophan
Tryptophan is one of the 20 standard amino acids, as well as an essential amino acid in the human diet. It is encoded in the standard genetic code as the codon UGG...
or endogenous Chlorophyll
Chlorophyll
Chlorophyll is a green pigment found in almost all plants, algae, and cyanobacteria. Its name is derived from the Greek words χλωρος, chloros and φύλλον, phyllon . Chlorophyll is an extremely important biomolecule, critical in photosynthesis, which allows plants to obtain energy from light...
, Phycoerythrin
Phycoerythrin
Phycoerythrin is a red protein from the light-harvesting phycobiliprotein family, present in cyanobacteria, red algae and cryptomonads.Like all phycobiliproteins, phycoerythrin is composed of a protein part, organised in a hexameric structure of alpha and beta chains, covalently binding...
or green fluorescent protein
Green fluorescent protein
The green fluorescent protein is a protein composed of 238 amino acid residues that exhibits bright green fluorescence when exposed to blue light. Although many other marine organisms have similar green fluorescent proteins, GFP traditionally refers to the protein first isolated from the...
). Alternatively, specific or general proteins, nucleic acid
Nucleic acid
Nucleic acids are biological molecules essential for life, and include DNA and RNA . Together with proteins, nucleic acids make up the most important macromolecules; each is found in abundance in all living things, where they function in encoding, transmitting and expressing genetic information...
s, lipids or small molecules can be "labelled" with an extrinsic fluorophore
Fluorophore
A fluorophore, in analogy to a chromophore, is a component of a molecule which causes a molecule to be fluorescent. It is a functional group in a molecule which will absorb energy of a specific wavelength and re-emit energy at a different wavelength...
, a fluorescent dye
Dye
A dye is a colored substance that has an affinity to the substrate to which it is being applied. The dye is generally applied in an aqueous solution, and requires a mordant to improve the fastness of the dye on the fiber....
which can be a small molecule, protein or quantum dot
Quantum dot
A quantum dot is a portion of matter whose excitons are confined in all three spatial dimensions. Consequently, such materials have electronic properties intermediate between those of bulk semiconductors and those of discrete molecules. They were discovered at the beginning of the 1980s by Alexei...
. Several techniques exist to exploit additional properties of fluorophore
Fluorophore
A fluorophore, in analogy to a chromophore, is a component of a molecule which causes a molecule to be fluorescent. It is a functional group in a molecule which will absorb energy of a specific wavelength and re-emit energy at a different wavelength...
s, such as fluorescence resonance energy transfer, where the energy is passed non-radiatively to a particular neighbouring dye, allowing proximity or protein activation to be detected; another is the change in proprieties, such as intensity, of certain dyes depending on their environment allowing their use in structural studies.
Fluorescence
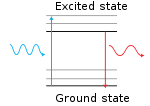
The principle behind fluorescence is that the fluorescent moiety
Fluorophore
A fluorophore, in analogy to a chromophore, is a component of a molecule which causes a molecule to be fluorescent. It is a functional group in a molecule which will absorb energy of a specific wavelength and re-emit energy at a different wavelength...
contains electrons which can absorb a photon
Photon
In physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force...
and briefly enter an excited state
Excited state
Excitation is an elevation in energy level above an arbitrary baseline energy state. In physics there is a specific technical definition for energy level which is often associated with an atom being excited to an excited state....
before either dispersing the energy non-radiatively or emitting it as a photon
Spontaneous emission
Spontaneous emission is the process by which a light source such as an atom, molecule, nanocrystal or nucleus in an excited state undergoes a transition to a state with a lower energy, e.g., the ground state and emits a photon...
, but with a lower energy, i.e., at a longer wavelength (wavelength and energy are inversely proportional).
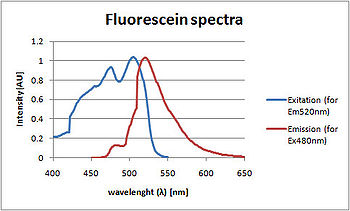
The difference in the excitation and emission wavelengths is called the Stokes shift
Stokes shift
Stokes shift is the difference between positions of the band maxima of the absorption and emission spectra of the same electronic transition. It is named after Irish physicist George G. Stokes. When a system absorbs a photon, it gains energy and enters an excited state...
, and the time that an excited electron takes to emit the photon is called a lifetime. The quantum yield
Quantum yield
The quantum yield of a radiation-induced process is the number of times that a defined event occurs per photon absorbed by the system. The "event" may represent a chemical reaction, for example the decomposition of a reactant molecule:...
is an indicator of the efficiency of the dye (it is the ratio of emitted photons per absorbed photon), and the extinction coefficient is the amount of light that can be absorbed by a fluorophore. Both the quantum yield and extinction coefficient are specific for each fluorophore and multiplied together calculates the brightness of the fluorescent molecule.
Reactive dyes
Fluorophores can be attached to proteins via specific functional groups, such as- amino groups (e.g. via succinimideSuccinimideSuccinimide is a cyclic imide with the formula C4H5NO2. It is used in a variety of organic syntheses, as well as in some industrial silver plating processes.-Succinimides:...
, IsothiocyanateIsothiocyanateIsothiocyanate is the chemical group –N=C=S, formed by substituting sulfur for oxygen in the isocyanate group. Many natural isothiocyanates from plants are produced by enzymatic conversion of metabolites called glucosinolates. These natural isothiocyanates, such as allyl isothiocyanate, are also...
or hydrazineHydrazineHydrazine is an inorganic compound with the formula N2H4. It is a colourless flammable liquid with an ammonia-like odor. Hydrazine is highly toxic and dangerously unstable unless handled in solution. Approximately 260,000 tons are manufactured annually...
) - carboxyl groups (e.g. via carbodiimideCarbodiimideA carbodiimide or a methanediimine is a functional group consisting of the formula RN=C=NR. Carbodiimides hydrolyze to form ureas, which makes them uncommon in nature.-Carbodiimide formation:...
) - thiolThiolIn organic chemistry, a thiol is an organosulfur compound that contains a carbon-bonded sulfhydryl group...
(e.g. via maleimideMaleimideMaleimide is the chemical compound with the formula H2C22NH . This unsaturated imide is an important building block in organic synthesis. The name is a contraction of maleic acid and imide, the -CNHC- functional group...
or acetyl bromideAcetyl bromideAcetyl bromide is an acyl bromide compound. As is expected, it may be prepared by reaction between phosphorus tribromide and acetic acid:...
) - azideAzideAzide is the anion with the formula N3−. It is the conjugate base of hydrazoic acid. N3− is a linear anion that is isoelectronic with CO2 and N2O. Per valence bond theory, azide can be described by several resonance structures, an important one being N−=N+=N−...
(e.g. via click chemistryClick chemistryClick chemistry is a chemical philosophy introduced by K. Barry Sharpless of The Scripps Research Institute, in 2001 and describes chemistry tailored to generate substances quickly and reliably by joining small units together...
)
or non-specificately (glutaraldehyde
Glutaraldehyde
Glutaraldehyde is an organic compound with the formula CH22. A pungent colorless oily liquid, glutaraldehyde is used to disinfect medical and dental equipment...
) or non-covalently (e.g. via hydrophobicity, etc).
These fluorophores are either small molecules, protein or quantum dots.
Organic fluorophores fluoresce thanks to delocalized electrons which can jump a band and stabilize the energy absorbed, hence most fluorophores are conjugated system
Conjugated system
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
s. Several families exits and their excitations range from the infrared
Infrared
Infrared light is electromagnetic radiation with a wavelength longer than that of visible light, measured from the nominal edge of visible red light at 0.74 micrometres , and extending conventionally to 300 µm...
to the ultraviolet
Ultraviolet
Ultraviolet light is electromagnetic radiation with a wavelength shorter than that of visible light, but longer than X-rays, in the range 10 nm to 400 nm, and energies from 3 eV to 124 eV...
.
Lanthanides (chelated) are uniquely fluorescent metals, which emit thanks to transitions involving 4f orbits, which are forbidden, hence they have very low absorption coefficients and slow emissions, requiring exitation through fluorescent organic chelators
Chelation
Chelation is the formation or presence of two or more separate coordinate bonds between apolydentate ligand and a single central atom....
(e.g. dipicolinate
Dipicolinic acid
Dipicolinic acid is a chemical compound which composes 5% to 15% of the dry weight of bacterial spores...
-based Terbium (III)
Terbium
Terbium is a chemical element with the symbol Tb and atomic number 65. It is a silvery-white rare earth metal that is malleable, ductile and soft enough to be cut with a knife...
chelators ).
A third class of small molecule fluorophore is that of the transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
-ligand complexes, which display molecular fluorescence from a metal-to-ligand charge transfer state which is partially forbidden, these are generally complexes of Ruthenium
Ruthenium
Ruthenium is a chemical element with symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most chemicals. The Russian scientist Karl Ernst Claus discovered the element...
, Rhenium
Rhenium
Rhenium is a chemical element with the symbol Re and atomic number 75. It is a silvery-white, heavy, third-row transition metal in group 7 of the periodic table. With an average concentration of 1 part per billion , rhenium is one of the rarest elements in the Earth's crust. The free element has...
or Osmium
Osmium
Osmium is a chemical element with the symbol Os and atomic number 76. Osmium is a hard, brittle, blue-gray or blue-blacktransition metal in the platinum family, and is the densest natural element. Osmium is twice as dense as lead. The density of osmium is , slightly greater than that of iridium,...
.
Fluorescent Proteins
Several fluorescent protein exist in nature, but the most important one as a research tool is Green Fluorescent ProteinGreen fluorescent protein
The green fluorescent protein is a protein composed of 238 amino acid residues that exhibits bright green fluorescence when exposed to blue light. Although many other marine organisms have similar green fluorescent proteins, GFP traditionally refers to the protein first isolated from the...
(GFP) from the jellyfish Aequorea victoria
Aequorea victoria
Aequorea victoria, also sometimes called the crystal jelly, is a bioluminescent hydrozoan jellyfish, or hydromedusa, that is found off the west coast of North America. This species is thought to be synonymous with Aequorea aequorea of Osamu Shimomura, the discoverer of green fluorescent protein . ...
, which spontaneously fluoresces upon folding via specific serine-tyrosine-glycine residues. The benefit that GFP and other fluorescent proteins have over organic dyes or quantum dots is that they can be expressed exogenously in cells alone or as a fusion protein
Fusion protein
Fusion proteins or chimeric proteins are proteins created through the joining of two or more genes which originally coded for separate proteins. Translation of this fusion gene results in a single polypeptide with functional properties derived from each of the original proteins...
, a protein that is created by ligating the fluorescent gene (e.g., GFP) to another gene and whose expression is driven by a housekeeping gene promoter or another specific promoter. This approach allows fluorescent proteins to be used as reporters for any number of biological events, such as sub-cellular localization and expression patterns
Spatiotemporal gene expression
Spatiotemporal gene expression is the activation of genes within specific tissues of an organism at specific times during development. Gene activation patterns vary widely in complexity. Some are straightforward and static, such as the pattern of tubulin, which is expressed in all cells at all...
.
A variant of GFP is naturally found in coral
Coral
Corals are marine animals in class Anthozoa of phylum Cnidaria typically living in compact colonies of many identical individual "polyps". The group includes the important reef builders that inhabit tropical oceans and secrete calcium carbonate to form a hard skeleton.A coral "head" is a colony of...
s, specifically the Anthozoa
Anthozoa
Anthozoa is a class within the phylum Cnidaria that contains the sea anemones and corals. Unlike other cnidarians, anthozoans do not have a medusa stage in their development. Instead, they release sperm and eggs that form a planula, which attaches to some substrate on which the cnidarian grows...
), and several mutants have been created to span the visible spectra and fluoresce longer and more stably.
Other proteins are fluorescent but require a fluorophore cofactor, and hence can only be used in vitro; these are often found in plants and algae (phytofluors, phycobiliprotein
Phycobiliprotein
Phycobiliproteins are water-soluble proteins present in cyanobacteria and certain algae that capture light energy, which is then passed on to chlorophylls during photosynthesis. Phycobiliproteins are formed of a complex between proteins and covalently bound phycobilins that act as chromophores...
such as allophycocyanin
Allophycocyanin
Allophycocyanin is a protein from the light-harvesting phycobiliprotein family, along with phycocyanin, phycoerythrin and phycoerythrocyanin. It is an accessory pigment to chlorophyll...
).
Bioluminescence and fluorescence
FluorescenceFluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation of a different wavelength. It is a form of luminescence. In most cases, emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation...
, chemiluminescence and phosphorescence
Phosphorescence
Phosphorescence is a specific type of photoluminescence related to fluorescence. Unlike fluorescence, a phosphorescent material does not immediately re-emit the radiation it absorbs. The slower time scales of the re-emission are associated with "forbidden" energy state transitions in quantum...
are 3 different types of luminescence
Luminescence
Luminescence is emission of light by a substance not resulting from heat; it is thus a form of cold body radiation. It can be caused by chemical reactions, electrical energy, subatomic motions, or stress on a crystal. This distinguishes luminescence from incandescence, which is light emitted by a...
properties, i.e. emission of light from a substance.
Fluorescence is a property where light is absorbed and remitted within a few nanoseconds (approx. 10ns) at a lower energy (=higher wavelength), while bioluminescence
Bioluminescence
Bioluminescence is the production and emission of light by a living organism. Its name is a hybrid word, originating from the Greek bios for "living" and the Latin lumen "light". Bioluminescence is a naturally occurring form of chemiluminescence where energy is released by a chemical reaction in...
is biological chemiluminescence, a property where light is generated by a chemical reaction of an enzyme on a substrate.
Phosphorescence
Phosphorescence
Phosphorescence is a specific type of photoluminescence related to fluorescence. Unlike fluorescence, a phosphorescent material does not immediately re-emit the radiation it absorbs. The slower time scales of the re-emission are associated with "forbidden" energy state transitions in quantum...
is a property of materials to absorb light and emit the energy several milliseconds or more later (due to forbidden transitions to the ground state
Ground state
The ground state of a quantum mechanical system is its lowest-energy state; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state...
of a triplet state
Triplet state
A spin triplet is a set of three quantum states of a system, each with total spin S = 1 . The system could consist of a single elementary massive spin 1 particle such as a W or Z boson, or be some multiparticle state with total spin angular momentum of one.In physics, spin is the angular momentum...
, while fluorescence occurs in exited singlet states). Until recently was not applicable to life science research due to the size of the inorganic particles. However the boundary between the fluorescence and phosphorescence is not clean cut as transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
-ligand complexes, which combine a metal and several organic moieties, have long lifetimes, up to several microseconds (as they display mixed singlet-triplet states).
Comparison with radioactivity
Prior to its widespread use in the past three decades radioactivity was the most common label.The advantages of fluorescence over radioactive labels are as follows:
- Fluorescence is much safer and more convenient to use
- Several fluorescent molecules can be used simultaneously given that they do not overlap, cf. FRET, whereas with radioactivity two isotopes can be used (tritiumTritiumTritium is a radioactive isotope of hydrogen. The nucleus of tritium contains one proton and two neutrons, whereas the nucleus of protium contains one proton and no neutrons...
and a low energy isotope such as 33P due to different intensities) but require special machinery (a tritium screen and a regular phosphor-imaging screen or a specific dual channel detector(e.g.). - Several properties are extremely useful (cf. next section)
Note: a channel
Channel (digital image)
Color digital images are made of pixels, and pixels are made of combinations of primary colors. A channel in this context is the grayscale image of the same size as a color image, made of just one of these primary colors. For instance, an image from a standard digital camera will have a red, green...
is similar to "colour" but distinct, it is the pair of excitation and emission filters specific for a dye, e.g. agilent microarrays are dual channel, working on cy3 and cy5, these are colloquially referred to as green and red.
Disadvantages of fluorophores include:
- Toxicity
- Interference with normal biological processes
Additional useful properties
The basic property of fluorescence are extensively used, such as a marker of labelled components in cells (fluorescence microscopy) or as an indicator in solution (Fluorescence spectroscopyFluorescence spectroscopy
Fluorescence spectroscopy aka fluorometry or spectrofluorometry, is a type of electromagnetic spectroscopy which analyzes fluorescence from a sample. It involves using a beam of light, usually ultraviolet light, that excites the electrons in molecules of certain compounds and causes them to emit...
), but other additional properties, not found with radioactivity, make it even more extensively used.
FRET

Dark quencher
A dark quencher is a substance that absorbs excitation energy from a fluorophore and dissipates the energy as heat; while a typical quencher re-emits much of this energy as light . Dark quenchers are used in molecular biology in conjunction with fluorophores...
or another fluorophore, which has an excitation spectrum which overlaps with the emission spectrum of the donor dye resulting in a reduced fluorescence.
This can be used to
- detect if two labelled protein or nucleic acids come into contact or a doubly labelled single molecules is hydrolysed
- detect changed in conformation
- measure concentration by a competitive binding assay
Sensitivity to environment

Indole
Indole is an aromatic heterocyclic organic compound. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing pyrrole ring. Indole is a popular component of fragrances and the precursor to many pharmaceuticals. Compounds that contain an...
, Cascade Yellow, prodan, Dansyl, Dapoxyl, NBD, PyMPO, Pyrene and diethylaminocumarin.
This change is most pronounced when electron-donating and electron-withdrawing groups are placed at opposite ends of an aromatic ring system, as this results in a large change in dipole moment
Electron electric dipole moment
The electron electric dipole moment de is an intrinsic property of an electron such that the potential energy is linearly related to the strength of the electric field: U=de·E. Within the standard model of elementary particle physics, such a dipole is predicted to be non-zero but very small, at...
when excited.
When a fluorophore is excited, it generally has a larger dipole moment (μE) than in the ground state (μG). Absorption of a photon by a fluorophore takes a few picoseconds. Before this energy is released (emission: 1–10 ns), the solvent molecules surrounding the fluorophore reorient (10–100 ps) due to the change in polarity in the excited singlet state; this process is called solvent relaxation. As a result of this relaxation, the energy of the excited state of the fluorophore is lowered (longer wavelength), hence fluorophores that have a large change in dipole moment have larger stokes shift changes in different solvents. The difference between the energy levels can be roughly determined with the Lipper-Mataga equation.
It should be noted that a hydrophobic dye is a dye which is insoluble in water, a property independent of solvatochromism.
Additionally, The term environment-sensitive
Chromism
In chemistry, chromism is a process that induces a change, often reversible, in the colors of compounds. In most cases, chromism is based on a change in the electron states of molecules, especially the π- or d-electron state, so this phenomenon is induced by various external stimuli which can alter...
in chemistry actually describes changes due to one of a variety of different environmental factors, such as pH or temperature, not just polarity; however, in biochemistry environment-sensitive fluorphore and solvatochromic fluorophore are used interchangeably: this convention is so widespread that suppliers describe them as environment-sensitive over solvatochromic ( example).
Fluorescence lifetime
Fluorescent moieties emit photons several nanoseconds after absorption following an exponential decay curve, which differs between dyes and depends on the surrounding solvent. When the dye is attached to a macromolecules the decay curve becomes multiexponential. Conjugated dyes generally have a lifetime between 1–10 ns, a small amount of longer lived exceptions exist, notably pyrene with a lifetime of 400ns in degassed solvents or 100ns in lipids and coroneneCoronene
Coronene is a polycyclic aromatic hydrocarbon comprising six peri-fused benzene rings. Its chemical formula is . It is a yellow material that dissolves in such solvents as benzene, toluene, and dichloromethane. Its solutions emit blue light fluorescence under UV light...
with 200ns. On a different category of fluorphores are the fluorescent organometals (lathanides and transition metal-ligand complexes) which have been previously described, which have much longer lifetimes due to the restricted states: lanthanides have lifetimes of 0.5 to 3 ms, while transition metal-ligand complexes have lifetimes of 10 ns to 10 µs. Note that fluorescent lifetime should not be confused with the photodestruction lifetime or the "shelf-life" of a dye.
Multiphoton excitation
Multiphoton excitation is a way of focusing the viewing plane of the microscope by taking advantage of the phenomenon where two simultaneous low energy photons are absorbed by a fluorescent moiety which normally absorbs one photon with double their individual energy: say two NIR photons (800 nm) to excite a UV dye (400 nm).Fluorescence anisotropy
A perfectly immobile fluorescent moiety when exited with polarized light will emit light which is also polarized. However if a molecule is moving, it will tend to "scramble" the polarization of the light by radiating at a different direction from the incident light.Methods
- Fluorescence microscopyFluorescence microscopeA fluorescence microscope is an optical microscope used to study properties of organic or inorganic substances using the phenomena of fluorescence and phosphorescence instead of, or in addition to, reflection and absorption...
of tissues, cells or subcellular structures is accomplished by labeling an antibody with a fluorophore and allowing the antibody to find its target antigen within the sample. Labeling multiple antibodies with different fluorophores allows visualization of multiple targets within a single image. - Automated sequencing of DNADNADeoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
by the chain termination method; each of four different chain terminating bases has its own specific fluorescent tag. As the labeled DNA molecules are separated, the fluorescent label is excited by a UV source, and the identity of the base terminating the molecule is identified by the wavelength of the emitted light.

- DNA detection: the compound ethidium bromideEthidium bromideEthidium bromide is an intercalating agent commonly used as a fluorescent tag in molecular biology laboratories for techniques such as agarose gel electrophoresis. It is commonly abbreviated as "EtBr", which is also an abbreviation for bromoethane...
, when free to change its conformation in solution, has very little fluorescence. Ethidium bromide's fluorescence is greatly enhanced when it binds to DNA, so this compound is very useful in visualising the location of DNA fragments in agarose gel electrophoresis. Ethidium bromide can be toxic – a safer alternative is the dye SYBR GreenSYBR GreenSYBR Green I is an asymmetrical cyanine dye used as a nucleic acid stain in molecular biology. SYBR Green I binds to DNA. The resulting DNA-dye-complex absorbs blue light and emits green light . The stain preferentially binds to double-stranded DNA, but will stain single-stranded DNA with lower...
. - The DNA microarrayDNA microarrayA DNA microarray is a collection of microscopic DNA spots attached to a solid surface. Scientists use DNA microarrays to measure the expression levels of large numbers of genes simultaneously or to genotype multiple regions of a genome...
- Immunology: An antibody has a fluorescent chemical group attached, and the sites (e.g., on a microscopic specimen) where the antibody has bound can be seen, and even quantified, by the fluorescence.
- FACS (fluorescent-activated cell sorting)
- Microscale ThermophoresisMicroscale ThermophoresisMicroscale Thermophoresis is a technology for the analysis of biomolecules. Microscale Thermophoresis is the directed movement of particles in a microscopic temperature gradient...
(MST) uses fluorescence as readout to quantify the directed movement of biomolecules in microscopic temperature gradients. - Fluorescence has been used to study the structure and conformations of DNA and proteins with techniques such as Fluorescence resonance energy transferFluorescence resonance energy transferFörster resonance energy transfer , also known as fluorescence resonance energy transfer, resonance energy transfer or electronic energy transfer , is a mechanism describing energy transfer between two chromophores.A donor chromophore, initially in its electronic excited state, may transfer energy...
, which measures distance at the angstrom level. This is especially important in complexes of multiple biomolecules. - Fluorescence can be applied to study colocalization of various proteins of interest. It then can be analyzed using a specialized software, like CoLocalizer ProCoLocalizer ProCoLocalizer Pro is a scientific software application, developed by , that allows researchers to analyze colocalization in the images obtained using fluorescence microscopy. Due to high popularity of Macintosh computers in medicine and biology, the software is designed specifically for Mac OS X...
.
Also, many biological molecules have an intrinsic fluorescence that can sometimes be used without the need to attach a chemical tag. Sometimes this intrinsic fluorescence changes when the molecule is in a specific environment, so the distribution or binding of the molecule can be measured. Bilirubin
Bilirubin
Bilirubin is the yellow breakdown product of normal heme catabolism. Heme is found in hemoglobin, a principal component of red blood cells. Bilirubin is excreted in bile and urine, and elevated levels may indicate certain diseases...
, for instance, is highly fluorescent when bound to a specific site on serum albumin. Zinc protoporphyrin
Zinc protoporphyrin
Zinc protoporphyrin is a compound found in red blood cells when heme production is inhibited by lead and/or by lack of iron. Instead of incorporating a ferrous ion, to form heme, protoporphyrin IX, the immediate precursor of heme, incorporates a zinc ion, forming ZPP...
, formed in developing red blood cells instead of hemoglobin when iron is unavailable or lead is present, has a bright fluorescence and can be used to detect these problems.
The number of fluorescence applications in the biomedical, biological and related sciences continuously expands. Methods of analysis in these fields are also growing, often with nomenclature in the form of acronyms such as: FLIM
Fluorescence lifetime imaging
Fluorescence-lifetime imaging microscopy or FLIM is an imaging technique for producing an image based on the differences in the exponential decay rate of the fluorescence from a fluorescent sample...
, FLI, FLIP
Fluorescence loss in photobleaching
Fluorescence Loss in Photobleaching, or FLIP, is a technique in fluorescence microscopy which can be used to examine the movement or diffusion of molecules inside cells or membranes. Typically a cell membrane is labelled with a fluorescent dye, and a specific area of the labeled membrane is...
, CALI, FLIE, FRET
Fluorescence resonance energy transfer
Förster resonance energy transfer , also known as fluorescence resonance energy transfer, resonance energy transfer or electronic energy transfer , is a mechanism describing energy transfer between two chromophores.A donor chromophore, initially in its electronic excited state, may transfer energy...
, FRAP
Fluorescence recovery after photobleaching
Fluorescence recovery after photobleaching denotes an optical technique capable of quantifying the two dimensional lateral diffusion of a molecularly thin film containing fluorescently labeled probes, or to examine single cells. This technique is very useful in biological studies of cell membrane...
, FCS
Fluorescence correlation spectroscopy
Fluorescence correlation spectroscopy is a correlation analysis of fluctuation of the fluorescence intensity. The analysis provides parameters of the physics under the fluctuations. One of the interesting applications of this is an analysis of the concentration fluctuations of fluorescent...
, PFRAP, smFRET, FIONA, FRIPS, SHREK, SHRIMP or TIRF
Total internal reflection fluorescence microscope
A total internal reflection fluorescence microscope is a type of microscope with which a thin region of a specimen, usually less than 200 nm, can be observed.-Background:...
. Most of these techniques rely on fluorescence microscopes, which use high intensity light sources, usually mercury or xenon lamps, LEDs, or lasers, to excite fluorescence in the samples under observation. Optical filters then separate excitation light from emitted fluorescence to be detected by eye or with a (CCD) camera or other light detector (e.g., photomultiplier tubes, spectrographs). Considerable research is underway to improve the capabilities of such microscopes, the fluorescent probes used, and the applications they are applied to. Of particular note are confocal microscopes, which use a pinhole to achieve optical sectioning
Optical sectioning
Optical sectioning is the process by which a suitably designed microscope can produce clear images of a focal planes deep within a thick sample. This is used to reduce the need for thin sectioning using instruments such as the microtome...
, which affords a quantitative, 3D view of the sample.

