Fluoride volatility
Encyclopedia
Fluoride volatility is jargon
that describes the volatility
of fluoride
s, which is relevant to the separation of radionuclide
s. Hexafluoride
s and pentafluorides have much lower boiling points than the lower-valence
fluorides. Most difluorides and trifluorides have high boiling points, while most tetrafluorides and monofluorides fall in between. The volatility of fluorides is the basis of technologies used in the processing and reprocessing
of nuclear fuel
, both of the conventional fuel rods used in today's LWRs and as a part of a molten salt reactor
system.
s react with fluorine to form gaseous uranium hexafluoride
, most of the plutonium
reacts to form gaseous plutonium hexafluoride, a majority of fission product
s (especially electropositive elements: lanthanide
s, strontium
, barium
, yttrium
, caesium
) form nonvolatile fluorides. Few metals in the fission products (the transition metal
s niobium
, ruthenium
, technetium
, molybdenum
, and the halogen
iodine
) form volatile (boiling point <200 °C) fluorides that accompany the uranium and plutonium hexafluorides, together with inert gas
es. Distillation
is then used to separate the uranium hexafluoride from the mixture.
The nonvolatile alkaline fission product
s and minor actinides
is most suitable for further processing with 'dry' electrochemical
processing (pyrochemical
) non-aqueous methods. The lanthanide
fluorides are difficult to dissolve in the nitric acid
used for aqueous reprocessing methods, such as PUREX
, DIAMEX and SANEX, which use solvent extraction. Fluoride volatility is only one of several pyrochemical processes designed to reprocess used nuclear fuel.
The Řež nuclear research institute at Řež
in the Czech Republic
tested screw dosers that fed ground uranium oxide (simulating used fuel pellets) into a fluorinator where the particles were burned in fluorine gas to form uranium hexafluoride
.
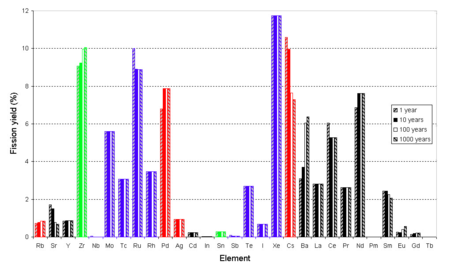 Valences for the majority of elements are based on the highest known fluoride.
Valences for the majority of elements are based on the highest known fluoride.
Roughly, fluoride volatility can be used to remove elements with a valence of 5 or greater: uranium
, neptunium
, plutonium
, metalloids (tellurium, antimony
), nonmetals (selenium
), halogens (iodine
, bromine
), and the middle transition metal
s (niobium
, molybdenum
, technetium
, ruthenium
, and possibly rhodium
). This fraction includes the actinides most easily reusable as nuclear fuel in a thermal reactor
, and the two long-lived fission product
s best suited to disposal by transmutation, Tc-99 and I-129
, as well as Se-79.
Noble gases (xenon
, krypton
) are volatile even without fluoridation, and will not condense except at much lower temperatures.
Left behind are alkali metals (caesium
, rubidium
), alkaline earth metals (strontium
, barium
), lanthanides, the remaining actinides (americium
, curium
), remaining transition metal
s (yttrium
, zirconium
, palladium
, silver
, cadmium
) and poor metals (tin
, indium
). This fraction contains the fission products that are radiation hazards on a scale of decades (Cs-137, Sr-90, Sm-151), the four remaining long-lived fission product
s Cs-135, Zr-93, Pd-107, Sn-126 of which only the last emits strong radiation, most of the neutron poisons, and the higher actinides (americium
, curium
, californium
) that are radiation hazards on a scale of hundreds or thousands of years and are difficult to work with because of gamma radiation but are fissionable in a fast reactor.
Missing top fluorides:
PrF4 (because it decomposes at 90 Celsius degrees)
TbF4 (because it decomposes at 300 Celsius degrees)
CeF4 (because it decomposes at 600 Celsius degrees)
Without stable fluorides: Kr 36, Xe 54, Pd 46
Jargon
Jargon is terminology which is especially defined in relationship to a specific activity, profession, group, or event. The philosophe Condillac observed in 1782 that "Every science requires a special language because every science has its own ideas." As a rationalist member of the Enlightenment he...
that describes the volatility
Volatility (chemistry)
In chemistry and physics, volatility is the tendency of a substance to vaporize. Volatility is directly related to a substance's vapor pressure. At a given temperature, a substance with higher vapor pressure vaporizes more readily than a substance with a lower vapor pressure.The term is primarily...
of fluoride
Fluoride
Fluoride is the anion F−, the reduced form of fluorine when as an ion and when bonded to another element. Both organofluorine compounds and inorganic fluorine containing compounds are called fluorides. Fluoride, like other halides, is a monovalent ion . Its compounds often have properties that are...
s, which is relevant to the separation of radionuclide
Radionuclide
A radionuclide is an atom with an unstable nucleus, which is a nucleus characterized by excess energy available to be imparted either to a newly created radiation particle within the nucleus or to an atomic electron. The radionuclide, in this process, undergoes radioactive decay, and emits gamma...
s. Hexafluoride
Hexafluoride
A hexafluoride is a chemical compound with the general formula XF6. Sixteen elements are known to form stable hexafluorides. Nine of these elements are transition metals, three are actinides, and four are nonmetals or metalloids.- Physical properties :...
s and pentafluorides have much lower boiling points than the lower-valence
Valence
-Chemistry and physics:* Valence * Valence bond theory in chemistry* Valence shell in chemistry* Valence band in physics* Valence quarks in particle physics-In other sciences:* Valency...
fluorides. Most difluorides and trifluorides have high boiling points, while most tetrafluorides and monofluorides fall in between. The volatility of fluorides is the basis of technologies used in the processing and reprocessing
Nuclear reprocessing
Nuclear reprocessing technology was developed to chemically separate and recover fissionable plutonium from irradiated nuclear fuel. Reprocessing serves multiple purposes, whose relative importance has changed over time. Originally reprocessing was used solely to extract plutonium for producing...
of nuclear fuel
Nuclear fuel
Nuclear fuel is a material that can be 'consumed' by fission or fusion to derive nuclear energy. Nuclear fuels are the most dense sources of energy available...
, both of the conventional fuel rods used in today's LWRs and as a part of a molten salt reactor
Molten salt reactor
A molten salt reactor is a type of nuclear fission reactor in which the primary coolant, or even the fuel itself is a molten salt mixture...
system.
Reprocessing methods
Uranium oxideUranium oxide
Uranium oxide is an oxide of the element uranium.The metal uranium forms several oxides:* Uranium dioxide or uranium oxide * Uranium trioxide or uranium oxide...
s react with fluorine to form gaseous uranium hexafluoride
Uranium hexafluoride
Uranium hexafluoride , referred to as "hex" in the nuclear industry, is a compound used in the uranium enrichment process that produces fuel for nuclear reactors and nuclear weapons. It forms solid grey crystals at standard temperature and pressure , is highly toxic, reacts violently with water...
, most of the plutonium
Plutonium
Plutonium is a transuranic radioactive chemical element with the chemical symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, forming a dull coating when oxidized. The element normally exhibits six allotropes and four oxidation...
reacts to form gaseous plutonium hexafluoride, a majority of fission product
Fission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus fissions. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons and a large release of energy in the form of heat , gamma rays and neutrinos. The...
s (especially electropositive elements: lanthanide
Lanthanide
The lanthanide or lanthanoid series comprises the fifteen metallic chemical elements with atomic numbers 57 through 71, from lanthanum through lutetium...
s, strontium
Strontium
Strontium is a chemical element with the symbol Sr and the atomic number 38. An alkaline earth metal, strontium is a soft silver-white or yellowish metallic element that is highly reactive chemically. The metal turns yellow when exposed to air. It occurs naturally in the minerals celestine and...
, barium
Barium
Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in Group 2, a soft silvery metallic alkaline earth metal. Barium is never found in nature in its pure form due to its reactivity with air. Its oxide is historically known as baryta but it reacts with...
, yttrium
Yttrium
Yttrium is a chemical element with symbol Y and atomic number 39. It is a silvery-metallic transition metal chemically similar to the lanthanides and it has often been classified as a "rare earth element". Yttrium is almost always found combined with the lanthanides in rare earth minerals and is...
, caesium
Caesium
Caesium or cesium is the chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-gold alkali metal with a melting point of 28 °C , which makes it one of only five elemental metals that are liquid at room temperature...
) form nonvolatile fluorides. Few metals in the fission products (the transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
s niobium
Niobium
Niobium or columbium , is a chemical element with the symbol Nb and atomic number 41. It's a soft, grey, ductile transition metal, which is often found in the pyrochlore mineral, the main commercial source for niobium, and columbite...
, ruthenium
Ruthenium
Ruthenium is a chemical element with symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most chemicals. The Russian scientist Karl Ernst Claus discovered the element...
, technetium
Technetium
Technetium is the chemical element with atomic number 43 and symbol Tc. It is the lowest atomic number element without any stable isotopes; every form of it is radioactive. Nearly all technetium is produced synthetically and only minute amounts are found in nature...
, molybdenum
Molybdenum
Molybdenum , is a Group 6 chemical element with the symbol Mo and atomic number 42. The name is from Neo-Latin Molybdaenum, from Ancient Greek , meaning lead, itself proposed as a loanword from Anatolian Luvian and Lydian languages, since its ores were confused with lead ores...
, and the halogen
Halogen
The halogens or halogen elements are a series of nonmetal elements from Group 17 IUPAC Style of the periodic table, comprising fluorine , chlorine , bromine , iodine , and astatine...
iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
) form volatile (boiling point <200 °C) fluorides that accompany the uranium and plutonium hexafluorides, together with inert gas
Inert gas
An inert gas is a non-reactive gas used during chemical synthesis, chemical analysis, or preservation of reactive materials. Inert gases are selected for specific settings for which they are functionally inert since the cost of the gas and the cost of purifying the gas are usually a consideration...
es. Distillation
Distillation
Distillation is a method of separating mixtures based on differences in volatilities of components in a boiling liquid mixture. Distillation is a unit operation, or a physical separation process, and not a chemical reaction....
is then used to separate the uranium hexafluoride from the mixture.
The nonvolatile alkaline fission product
Fission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus fissions. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons and a large release of energy in the form of heat , gamma rays and neutrinos. The...
s and minor actinides
Minor actinides
The minor actinides are the actinide elements in used nuclear fuel other than uranium and plutonium, which are termed the major actinides. The minor actinides include neptunium, americium, curium, berkelium, californium, einsteinium, and fermium...
is most suitable for further processing with 'dry' electrochemical
Electrochemistry
Electrochemistry is a branch of chemistry that studies chemical reactions which take place in a solution at the interface of an electron conductor and an ionic conductor , and which involve electron transfer between the electrode and the electrolyte or species in solution.If a chemical reaction is...
processing (pyrochemical
Pyroprocessing
Pyroprocessing is a process in which materials are subjected to high temperatures in order to bring about a chemical or physical change. Pyroprocessing includes such terms as ore-roasting, calcination and sintering...
) non-aqueous methods. The lanthanide
Lanthanide
The lanthanide or lanthanoid series comprises the fifteen metallic chemical elements with atomic numbers 57 through 71, from lanthanum through lutetium...
fluorides are difficult to dissolve in the nitric acid
Nitric acid
Nitric acid , also known as aqua fortis and spirit of nitre, is a highly corrosive and toxic strong acid.Colorless when pure, older samples tend to acquire a yellow cast due to the accumulation of oxides of nitrogen. If the solution contains more than 86% nitric acid, it is referred to as fuming...
used for aqueous reprocessing methods, such as PUREX
PUREX
PUREX is an acronym standing for Plutonium - URanium EXtraction — de facto standard aqueous nuclear reprocessing method for the recovery of uranium and plutonium from used nuclear fuel. It is based on liquid-liquid extraction ion-exchange.The PUREX process was invented by Herbert H. Anderson and...
, DIAMEX and SANEX, which use solvent extraction. Fluoride volatility is only one of several pyrochemical processes designed to reprocess used nuclear fuel.
The Řež nuclear research institute at Řež
Rez
Rez, developed under the codename K-Project, Project Eden, and Vibes, is a rail shooter video game released by Sega in Japan in 2001 for the Dreamcast and PlayStation 2, with a European Dreamcast release and United States PlayStation 2 release in 2002...
in the Czech Republic
Czech Republic
The Czech Republic is a landlocked country in Central Europe. The country is bordered by Poland to the northeast, Slovakia to the east, Austria to the south, and Germany to the west and northwest....
tested screw dosers that fed ground uranium oxide (simulating used fuel pellets) into a fluorinator where the particles were burned in fluorine gas to form uranium hexafluoride
Uranium hexafluoride
Uranium hexafluoride , referred to as "hex" in the nuclear industry, is a compound used in the uranium enrichment process that produces fuel for nuclear reactors and nuclear weapons. It forms solid grey crystals at standard temperature and pressure , is highly toxic, reacts violently with water...
.
Volatility and valence

Roughly, fluoride volatility can be used to remove elements with a valence of 5 or greater: uranium
Uranium
Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
, neptunium
Neptunium
Neptunium is a chemical element with the symbol Np and atomic number 93. A radioactive metal, neptunium is the first transuranic element and belongs to the actinide series. Its most stable isotope, 237Np, is a by-product of nuclear reactors and plutonium production and it can be used as a...
, plutonium
Plutonium
Plutonium is a transuranic radioactive chemical element with the chemical symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, forming a dull coating when oxidized. The element normally exhibits six allotropes and four oxidation...
, metalloids (tellurium, antimony
Antimony
Antimony is a toxic chemical element with the symbol Sb and an atomic number of 51. A lustrous grey metalloid, it is found in nature mainly as the sulfide mineral stibnite...
), nonmetals (selenium
Selenium
Selenium is a chemical element with atomic number 34, chemical symbol Se, and an atomic mass of 78.96. It is a nonmetal, whose properties are intermediate between those of adjacent chalcogen elements sulfur and tellurium...
), halogens (iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
, bromine
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
), and the middle transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
s (niobium
Niobium
Niobium or columbium , is a chemical element with the symbol Nb and atomic number 41. It's a soft, grey, ductile transition metal, which is often found in the pyrochlore mineral, the main commercial source for niobium, and columbite...
, molybdenum
Molybdenum
Molybdenum , is a Group 6 chemical element with the symbol Mo and atomic number 42. The name is from Neo-Latin Molybdaenum, from Ancient Greek , meaning lead, itself proposed as a loanword from Anatolian Luvian and Lydian languages, since its ores were confused with lead ores...
, technetium
Technetium
Technetium is the chemical element with atomic number 43 and symbol Tc. It is the lowest atomic number element without any stable isotopes; every form of it is radioactive. Nearly all technetium is produced synthetically and only minute amounts are found in nature...
, ruthenium
Ruthenium
Ruthenium is a chemical element with symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most chemicals. The Russian scientist Karl Ernst Claus discovered the element...
, and possibly rhodium
Rhodium
Rhodium is a chemical element that is a rare, silvery-white, hard and chemically inert transition metal and a member of the platinum group. It has the chemical symbol Rh and atomic number 45. It is composed of only one isotope, 103Rh. Naturally occurring rhodium is found as the free metal, alloyed...
). This fraction includes the actinides most easily reusable as nuclear fuel in a thermal reactor
Thermal reactor
A thermal reactor is a nuclear reactor that uses slow or thermal neutrons. Most power reactors are of this type. These type of reactors use a neutron moderator to slow neutrons until they approach the average kinetic energy of the surrounding particles, that is, to reduce the speed of the neutrons...
, and the two long-lived fission product
Long-lived fission product
Long-lived fission products are radioactive materials with a long half-life produced by nuclear fission.-Evolution of radioactivity in nuclear waste:...
s best suited to disposal by transmutation, Tc-99 and I-129
Iodine-129
Iodine-129 is long-lived radioisotope of iodine which occurs naturally, but also is of special interest in the monitoring and effects of man-made nuclear fission decay products, where it serves as both tracer and potential radiological contaminant....
, as well as Se-79.
Noble gases (xenon
Xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. The element name is pronounced or . A colorless, heavy, odorless noble gas, xenon occurs in the Earth's atmosphere in trace amounts...
, krypton
Krypton
Krypton is a chemical element with the symbol Kr and atomic number 36. It is a member of Group 18 and Period 4 elements. A colorless, odorless, tasteless noble gas, krypton occurs in trace amounts in the atmosphere, is isolated by fractionally distilling liquified air, and is often used with other...
) are volatile even without fluoridation, and will not condense except at much lower temperatures.
Left behind are alkali metals (caesium
Caesium
Caesium or cesium is the chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-gold alkali metal with a melting point of 28 °C , which makes it one of only five elemental metals that are liquid at room temperature...
, rubidium
Rubidium
Rubidium is a chemical element with the symbol Rb and atomic number 37. Rubidium is a soft, silvery-white metallic element of the alkali metal group. Its atomic mass is 85.4678. Elemental rubidium is highly reactive, with properties similar to those of other elements in group 1, such as very rapid...
), alkaline earth metals (strontium
Strontium
Strontium is a chemical element with the symbol Sr and the atomic number 38. An alkaline earth metal, strontium is a soft silver-white or yellowish metallic element that is highly reactive chemically. The metal turns yellow when exposed to air. It occurs naturally in the minerals celestine and...
, barium
Barium
Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in Group 2, a soft silvery metallic alkaline earth metal. Barium is never found in nature in its pure form due to its reactivity with air. Its oxide is historically known as baryta but it reacts with...
), lanthanides, the remaining actinides (americium
Americium
Americium is a synthetic element that has the symbol Am and atomic number 95. This transuranic element of the actinide series is located in the periodic table below the lanthanide element europium, and thus by analogy was named after another continent, America.Americium was first produced in 1944...
, curium
Curium
Curium is a synthetic chemical element with the symbol Cm and atomic number 96. This radioactive transuranic element of the actinide series was named after Marie Skłodowska-Curie and her husband Pierre Curie. Curium was first intentionally produced and identified in summer 1944 by the group of...
), remaining transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
s (yttrium
Yttrium
Yttrium is a chemical element with symbol Y and atomic number 39. It is a silvery-metallic transition metal chemically similar to the lanthanides and it has often been classified as a "rare earth element". Yttrium is almost always found combined with the lanthanides in rare earth minerals and is...
, zirconium
Zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name of zirconium is taken from the mineral zircon. Its atomic mass is 91.224. It is a lustrous, grey-white, strong transition metal that resembles titanium...
, palladium
Palladium
Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
, silver
Silver
Silver is a metallic chemical element with the chemical symbol Ag and atomic number 47. A soft, white, lustrous transition metal, it has the highest electrical conductivity of any element and the highest thermal conductivity of any metal...
, cadmium
Cadmium
Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, bluish-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Similar to zinc, it prefers oxidation state +2 in most of its compounds and similar to mercury it shows a low...
) and poor metals (tin
Tin
Tin is a chemical element with the symbol Sn and atomic number 50. It is a main group metal in group 14 of the periodic table. Tin shows chemical similarity to both neighboring group 14 elements, germanium and lead and has two possible oxidation states, +2 and the slightly more stable +4...
, indium
Indium
Indium is a chemical element with the symbol In and atomic number 49. This rare, very soft, malleable and easily fusible post-transition metal is chemically similar to gallium and thallium, and shows the intermediate properties between these two...
). This fraction contains the fission products that are radiation hazards on a scale of decades (Cs-137, Sr-90, Sm-151), the four remaining long-lived fission product
Long-lived fission product
Long-lived fission products are radioactive materials with a long half-life produced by nuclear fission.-Evolution of radioactivity in nuclear waste:...
s Cs-135, Zr-93, Pd-107, Sn-126 of which only the last emits strong radiation, most of the neutron poisons, and the higher actinides (americium
Americium
Americium is a synthetic element that has the symbol Am and atomic number 95. This transuranic element of the actinide series is located in the periodic table below the lanthanide element europium, and thus by analogy was named after another continent, America.Americium was first produced in 1944...
, curium
Curium
Curium is a synthetic chemical element with the symbol Cm and atomic number 96. This radioactive transuranic element of the actinide series was named after Marie Skłodowska-Curie and her husband Pierre Curie. Curium was first intentionally produced and identified in summer 1944 by the group of...
, californium
Californium
Californium is a radioactive metallic chemical element with the symbol Cf and atomic number 98. The element was first made in the laboratory in 1950 by bombarding curium with alpha particles at the University of California, Berkeley. It is the ninth member of the actinide series and was the...
) that are radiation hazards on a scale of hundreds or thousands of years and are difficult to work with because of gamma radiation but are fissionable in a fast reactor.
Fluorides by boiling and melting points
| Fluoride | Z Atomic number In chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element... | Boiling Boiling point The boiling point of an element or a substance is the temperature at which the vapor pressure of the liquid equals the environmental pressure surrounding the liquid.... °C | Melting Melting point The melting point of a solid is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at standard atmospheric pressure... °C | Key halflife | Yield Fission product yield Nuclear fission splits a heavy nucleus such as uranium or plutonium into two lighter nuclei, which are called fission products. Yield refers to the fraction of a fission product produced per fission.Yield can be broken down by:#Individual isotope... |
|---|---|---|---|---|---|
| SeF6 Selenium hexafluoride Selenium hexafluoride is the inorganic compound with the formula SeF6. It is a colourless gas described as having a "repulsive" odor. It is not widely encountered and has no commercial applications.-Structure, preparation, and reactions:... |
34 | −46.6 | −50.8 | 79Se:65ky | .04% |
| TeF6 Tellurium hexafluoride Tellurium hexafluoride is the oldest known fluoride of tellurium. It is a colorless, highly toxic gas with an extremely unpleasant smell.-Preparation:... |
52 | −39 | −38 | 127mTe:109d | |
| IF7 Iodine heptafluoride Iodine heptafluoride, also known as iodine fluoride or even iodine fluoride, is an interhalogen compound with chemical formula IF7. It has an unusual pentagonal bipyramidal structure, as predicted by VSEPR theory... |
53 | 4.8 (1 atm) | 6.5 (tripoint Tripoint A tripoint, or trijunction , is a geographical point at which the borders of three countries or subnational entities meet.... ) |
129I:15.7my | 0.54% |
| MoF6 Molybdenum hexafluoride Molybdenum hexafluoride is the highest fluoride of molybdenum. It is a solid which melts just below room temperature; in water, it hydrolyses to give hydrofluoric acid.... |
42 | 34 | 17.4 | 99Mo:2.75d | |
| PuF6 Plutonium hexafluoride Plutonium hexafluoride is the highest fluoride of plutonium, and is of interest for laser enrichment of plutonium, in particular for the production of pure plutonium-239 from irradiated uranium... |
94 | 52 (subl) | 62 | 239Pu:24ky | |
| TcF6 Technetium(VI) fluoride Technetium fluoride is a yellow inorganic compound with a low melting point. It was first identified in 1961. In this compound, technetium has an oxidation state of +6, the highest oxidation state found in the technetium halides. The other such compound is technetium chloride, TcCl6... |
43 | 55.3 | 37.4 | 99Tc:213ky | 6.1% |
| NpF6 | 93 | 55.18 | 54.4 | 237Np:2.14my | |
| UF6 Uranium hexafluoride Uranium hexafluoride , referred to as "hex" in the nuclear industry, is a compound used in the uranium enrichment process that produces fuel for nuclear reactors and nuclear weapons. It forms solid grey crystals at standard temperature and pressure , is highly toxic, reacts violently with water... |
92 | 56.5 (subl) | 64.8 | 233U Uranium-233 Uranium-233 is a fissile isotope of uranium, bred from Thorium as part of the thorium fuel cycle. It has been used in a few nuclear reactors and has been proposed for much wider use as a nuclear fuel. It has a half-life of 160,000 years.... :160ky |
|
| ReF7 Rhenium heptafluoride Rhenium heptafluoride is the compound with the formula ReF7. It is a yellow low melting solid, and is the only thermally stable metal heptafluoride. It has a distorted pentagonal bipyramidal structure similar to IF7, which was confirmed by neutron diffraction at 1.5K. The structure is non-rigid as... |
75 | 73.72 | 48.3 | Not FP | |
| BrF5 Bromine pentafluoride Bromine pentafluoride, BrF5, is an interhalogen compound and a fluoride of bromine. It is a strong fluorination reagent.It melts at −61.30 °C and boils at 40.25 °C. BrF5 finds use in oxygen isotope analysis. Laser ablation of solid silicates in the presence of bromine pentafluoride releases O2 for... |
35 | 40.25 | −61.30 | 81Br:stable | |
| IF5 Iodine pentafluoride Iodine pentafluoride is an interhalogen compound with chemical formula IF5. It is a fluoride of iodine. It is a colourless or yellow liquid with a density of 3.250 g cm−3. It was first synthesized by Henri Moissan in 1891 by burning solid iodine in fluorine gas... |
53 | 97.85 | 9.43 | 129I:15.7my | 0.54% |
| SbF5 Antimony pentafluoride Antimony pentafluoride is the inorganic compound with the formula SbF5. This colourless, viscous liquid is a valuable Lewis acid and a component of the superacid fluoroantimonic acid, the strongest known acid... |
51 | 141 | 8.3 | 125Sb:2.76y | |
| RuOF4 | 44 | 184 | 115 | 106Ru:374d | |
| RuF5 | 44 | 227 | 86.5 | 106Ru:374d | |
| NbF5 | 41 | 234 | 79 | 95Nb:35d | low |
| SnF4 | 50 | 705 | 750 (subl) | 121m1Sn:44y 126Sn:230ky |
0.013% ? |
| ZrF4 | 40 | 905 | 932 (tripoint) | 93Zr:1.5my | 6.35% |
| AgF | 47 | 1159 | 435 | 109Ag:stable | |
| CsF Caesium fluoride Caesium fluoride , is an inorganic compound usually encountered as a hygroscopic white solid. It is more soluble and more readily dissociated than sodium fluoride or potassium fluoride. It is available in anhydrous form, and if water has been absorbed it is easy to dry by heating at 100 °C for... |
55 | 1251 | 682 | 137Cs Caesium-137 Caesium-137 is a radioactive isotope of caesium which is formed as a fission product by nuclear fission.It has a half-life of about 30.17 years, and decays by beta emission to a metastable nuclear isomer of barium-137: barium-137m . Caesium-137 is a radioactive isotope of caesium which is formed... :30.2y 135Cs:2.3my |
6.19% 6.54% |
| BeF2 Beryllium fluoride Beryllium fluoride is the inorganic compound with the formula BeF2. This white solid is the principal precursor for the manufacture of beryllium metal.-Structure and bonding:... |
4 | 1327 | 552 | ||
| RbF Rubidium fluoride Rubidium fluoride is the fluoride salt of rubidium. It is an octahedral crystal.There are several methods for synthesising rubidium fluoride... |
37 | 1410 | 795 | ||
| UF4 Uranium tetrafluoride Uranium tetrafluoride is a green crystalline solid compound of uranium with an insignificant vapor pressure and very slight solubility in water. Uranium in its tetravalent state is very important in different technological processes... |
92 | 1417 | 1036 | U-233 Uranium-233 Uranium-233 is a fissile isotope of uranium, bred from Thorium as part of the thorium fuel cycle. It has been used in a few nuclear reactors and has been proposed for much wider use as a nuclear fuel. It has a half-life of 160,000 years.... :160ky |
|
| FLiBe FLiBe FLiBe is a mixture of lithium fluoride and beryllium fluoride . As a molten salt it is proposed as a nuclear reactor coolant, and two different mixtures were used in the Molten-Salt Reactor Experiment.... |
1430 | 459 | stable | ||
| FLiNaK FLiNaK FLiNaK is the name of the ternary eutectic alkaline metal fluoride salt mixture LiF-NaF-KF . It has a melting point of 454 °C and a boiling point of 1570 °C. It is used as electrolyte for the electroplating of refractory metals and compounds like titanium, tantalum, hafnium, zirconium and their... |
1570 | 454 | stable | ||
| LiF Lithium fluoride Lithium fluoride is an inorganic compound with the formula LiF. It is the lithium salt of hydrofluoric acid. This white solid is a simple ionic compound. Its structure is analogous to that of sodium chloride, but it is much less soluble in water. It is mainly used as a component of molten... |
3 | 1676 | 848 | stable | |
| ThF4 Thorium(IV) fluoride Thorium fluoride is an inorganic chemical compound. It is a white, hygroscopic powder which can be produced by reacting thorium with fluorine gas. At temperatures above 500 °C, it reacts with atmospheric moisture to produce ThOF2.-Uses:... |
90 | 1680 | 1110 | ||
| CdF2 Cadmium fluoride Cadmium fluoride is a mostly water-insoluble source of cadmium used in oxygen-sensitive applications, such as the production of metallic alloys. In extremely low concentrations , this and other fluoride compounds are used in limited medical treatment protocols... |
48 | 1748 | 1110 | 113mCd:14.1y | |
| YF3 | 39 | 2230 | 1150 | 91Y:58.51d | |
| InF3 Indium halides There are three sets of indium halides, the trihalides, the monohalides, and several intermediate halides.In the monohalides the oxidation state of indium is +1 and their proper names are indium fluoride, indium chloride, indium bromide and indium iodide.The intermediate halides contain indium with... |
49 | >1200 | 1170 | ||
| BaF2 Barium fluoride Barium fluoride is a chemical compound of barium and fluorine. It is a solid which can be a transparent crystal. It occurs in nature as the mineral frankdicksonite.-Structure:... |
56 | 2260 | 1368 | 140Ba:12.75d | |
| TbF3 | 65 | 2280 | 1172 | ||
| GdF3 | 64 | 1231 | 159Gd:18.5h | ||
| PmF3 | 61 | 1338 | 147Pm:2.62y | ||
| EuF3 | 63 | 2280 | 1390 | 155Eu:4.76y | |
| NdF3 | 60 | 2300 | 1374 | 147Nd:11d | |
| PrF3 | 59 | 1395 | 143Pr:13.57d | ||
| CeF3 | 58 | 2327 | 1430 | 144Ce:285d | |
| SmF3 | 62 | 2427 | 1306 | 151Sm:90y | 0.419% ? |
| SrF2 Strontium fluoride Strontium fluoride, SrF2, also called strontium difluoride and strontium fluoride, is a fluoride of strontium. It is a stable brittle white crystalline solid with melting point of 1477°C and boiling point 2460°C.-Preparation:... |
38 | 2460 | 1477 | 90Sr Strontium-90 Strontium-90 is a radioactive isotope of strontium, with a half-life of 28.8 years.-Radioactivity:Natural strontium is nonradioactive and nontoxic, but 90Sr is a radioactivity hazard... : 29.1y |
5.8% |
| LaF3 | 57 | 1493 | 140La:1.68d |
Missing top fluorides:
PrF4 (because it decomposes at 90 Celsius degrees)
TbF4 (because it decomposes at 300 Celsius degrees)
CeF4 (because it decomposes at 600 Celsius degrees)
Without stable fluorides: Kr 36, Xe 54, Pd 46
External links
- STUDY OF ELECTROCHEMICAL PROCESSES FOR SEPARATION OF THE ACTINIDES AND LANTHANIDES IN MOLTEN FLUORIDE MEDIA (PDF)
- Separation and purification of UF6 from volatile fluorides by rectification (PDF)
- Low-pressure distillation of a portion of the fuel carrier salt from the Molten Salt Reactor Experiment (PDF)
- USE OF THE FLUORIDE VOLATILITY PROCESS TO EXTRACT TECHNETIUM FROM TRANSMUTED SPENT NUCLEAR FUEL (PDF)
- A Peer Review of the Strategy for Characterizing Transuranics and Technetium Contamination in Depleted Uranium Hexafluoride Tails Cylinders (PDF)
- PHYSICAL CONSTANTS OF INORGANIC COMPOUNDS (PDF)

