Organophosphorus
Encyclopedia
Organophosphorus compounds are degradable organic compound
s containing carbon
–phosphorus
bonds
(thus excluding from phosphate and phosphite ester
s, which lack such kind of bonding), used primarily in pest control
as an alternative to chlorinated hydrocarbons that persist in the environment. Organophosphorus chemistry is the corresponding science of the properties and reactivity of organophosphorus compounds. Phosphorus shares group 5
in the periodic table with nitrogen
and phosphorus compounds and nitrogen compounds are somewhat related.
The definition of organophosphorus compounds is variable, which can lead to confusion. In industrial and environmental chemistry, an organophosphorus compound need contain only an organic substituent, but need not have a direct P-C bond. Thus most pesticides, e.g., malathion
, are often included in this class of compounds.
Phosphorus can adopt a variety of oxidation state
s, and it is general to classify organophosphorus compounds based on their being derivatives of phosphorus(V) vs phosphorus(III), which are the predominant classes of compounds. In a descriptive but only intermittently used nomenclature, phosphorus compounds are identified by their coordination number
δ
and their valency
λ
. In this system, a phosphine is a δ3λ3 compound.
agents, and plasticizer
s. Lacking a P−C bond, these compounds are in the technical sense not organophosphorus compounds but esters of phosphoric acid. Many derivatives are found in nature, such as phosphatidylcholine
. Phosphate ester are synthesized by alcoholysis of phosphorus oxychloride. A variety of mixed amido-alkoxo derivatives are known, one medically significant example being the anti-cancer drug cyclophosphamide
. Also derivatives containing the thiophosphoryl group (P=S) include the pesticide malathion
. The organophosphates prepared on the largest scale are the zinc dithiophosphates, as additives for motor oil. Several million kilograms of this coordination complex are produced annually by the reaction of phosphorus pentasulfide with alcohols.
In the environment, these compounds break down via hydrolysis
to eventually afford phosphate
and the organic alcohol or amine from which they are derived.
s are esters of phosphonic acid and have the general formula RP(=O)(OR')2. Phosphonates have many technical applications, a well-known member being Glyphosate, better known as Roundup. With the formula (HO)2P(O)CH2NHCH2CO2H, this derivative of glycine
is one of the most widely used herbicides. Bisphosphonate
s are a class of drugs to treat osteoporosis
. The nerve gas agent Sarin
, containing both C–P and F–P bonds, is a phosphonate.
Phosphinates feature two P–C bonds, with the general formula R2P(=O)(OR'). A commercially significant member is the herbicide Glufosinate
. Similar to Glyphosate mentioned above, it has the structure CH3P(O)(OH)CH2CH2CH(NH2)CO2H.
The Michaelis–Arbuzov reaction is the main method for the synthesis of these compounds. For example, dimethylmethylphosphonate (see figure above) arises from the rearrangement of trimethylphosphite, which is catalyzed by methyl iodide. In the Horner–Wadsworth–Emmons reaction and the Seyferth–Gilbert homologation, phosphonates are used in reactions with carbonyl
compounds. The Kabachnik–Fields reaction is a method for the preparation of aminophosphonates. These compounds contain a very inert bond between phosphorus and carbon. Consequently, they hydrolyze to give phosphonic and phosphinic acid derivatives, but not phosphate.
s and some are therefore soluble in water. The P=O bond is very polar with a dipole moment of 4.51 D for triphenylphosphine oxide
.
Compounds related to phosphine oxides are the imide
s (R3PNR') and related chalcogenide
s (R3PE, where E = S
, Se
, Te). These compounds are some of the most thermally stable organophosphorus compounds, but few are useful in significant amounts.
. These species are tetrahedral phosphorus(V) compounds. From the commercial perspective, the most important member is tetrakis(hydroxymethyl)phosphonium chloride
, [P(CH2OH)4]Cl, which is used as a fire retardant in textile
s. Approximately 2M kg are produced annually of the chloride and the related sulfate. They are generated by the reaction of phosphine with formaldehyde
in the presence of the mineral acid:
A variety of phosphonium salts can be prepared by alkylation
and arylation of organophosphines:
The methylation of triphenylphosphine is the first step in the preparation of the Wittig reagent.
The parent phosphorane
(δ5λ5) is PH5, which is unknown. Related compounds containing both halide and organic substituents on phosphorus are fairly common. Those with five organic substituents are rare, although P(C6H5)5 is known, being derived from P(C6H5)4+ by reaction with phenyllithium
.
Phosphorus ylide
s are unsaturated phosphoranes, known as Wittig reagents, e.g. CH2P(C6H5)3. These compounds feature tetrahedral phosphorus(V) and are considered relatives of phosphine oxides. They also are derived from phosphonium salts, but by deprotonation not alkylation.
s, have the general structure P(OR)3 with oxidation state +3. Such species arise from the alcoholysis of phosphorus trichloride:
The reaction is general, thus a vast number of such species are known. Phosphites are employed in the Perkow reaction
and the Michaelis–Arbuzov reaction. They also serve as ligands in organometallic chemistry.
Intermediate between phosphites and phosphines are phosphonite
s (P(OR)2R') and phosphinite
(P(OR)R'2). Such species arise via alcoholysis reactions of the corresponding phosphinous and phosphonous chlorides ((PClR'2) and PCl2R', respectively).
in the US and British Commonwealth, but phosphane elsewhere. Replacement of one or more hydrogen centers by an organic substituents (alkyl, aryl), gives PH3−xRx, an organophosphine, generally referred to as phosphines.
s. Like amines, phosphines have a trigonal pyramidal molecular geometry although often with smaller C-E-C angles (E = N, P), at least in the absence of steric effects. The C-P-C bond angle is 98.6° for trimethylphosphine increasing to 109.7° when the methyl groups are replaced by tert-butyl groups. When used as ligands, the steric bulk of tertiary phosphines is evaluated by their cone angle
. The barrier to inversion is also much higher than in amines for a process like nitrogen inversion
to occur, and therefore phosphines with three different substituent
s can be resolved into thermally stable optical isomers. Phosphines are often less basic than corresponding amines, for instance the phosphonium ion itself has a pKa
of −14 compared to 9.21 for the ammonium ion; trimethylphosphonium
has a pKa
of 8.65 compared to 9.76 for trimethylamine
. However, triphenylphosphine (pKa 2.73) is more basic than triphenylamine
(pKa −5), mainly because the lone pair of the nitrogen in NPh3 is partially delocalized into the three phenyl rings. Whereas the lone pair on nitrogen is delocalized in pyrrole
, the lone pair on phosphorus atom in the phosphorus equivalent of pyrrole
(phosphole
) is not. The reactivity of phosphines matches that of amines with regard to nucleophilicity in the formation of phosphonium salt
s with the general structure PR4+X−. This property is used in the Appel reaction
for converting alcohol
s to alkyl halides. Phosphines are easily oxidized to the corresponding phosphine oxide
s, whereas amine oxides are less readily generated. In part for this reason, phosphines are very rarely encountered in nature.
, several million kilograms being produced annually. It is prepared from the reaction of chlorobenzene
, PCl3
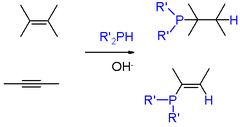
, and sodium. Phosphines of a more specialized nature are usually prepared by other routes. Phosphorus halides undergo Nucleophilic displacement by organometallic reagents such as Grignard reagents. Conversely, some syntheses entail nucleophilic displacement of phosphide anion equivalents ("R2P-") by aryl- and alkyl halides. Primary (RPH2) and secondary phosphines (RRPH and R2PH) add to alkene
s in presence of a strong base (e.g., KOH
in DMSO
). Markovnikov's rule
s apply. Similar reactions occur involving alkyne
s. Base is not required for electron-deficient alkenes (e.g., derivatives of acrylonitrile
) and alkynes.
Under free-radical conditions the P-H bonds of primary and secondary phosphines add across alkenes. Such reactions proceed with anti-Markovnikov regiochemistry. AIBN or organic peroxide
s are used as initiator
s. Tertiary phosphine oxides and sulfides can be reduced with chlorosilane
s and other reagents.
s. Phosphines are nucleophilic catalysts in the dimerization of enones in various reactions in organic synthesis
, e.g. the Rauhut–Currier reaction.
Phosphines are reducing agent
s, as illustrated in the Staudinger reduction converting azides to amines and in the Mitsunobu reaction
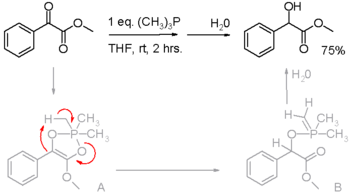
for converting alcohols into esters. In these processes, the phosphine is oxidized to the phosphine oxide. Phosphines have also been found to reduce activated carbonyl groups for instance the reduction of an α-keto ester to an α-hydroxy ester in scheme 2. In the proposed reaction mechanism
, the first proton is on loan from the methyl group in trimethylphosphine (triphenylphosphine does not react).
are important ligand
s in inorganic chemistry. Mainly owing to the utility of asymmetric synthesis, a variety of chiral diphosphines
have been popularized, such as BINAP
and DIPAMP
. A large number of phosphine ligands including diphosphines are simply called "phos ligands".
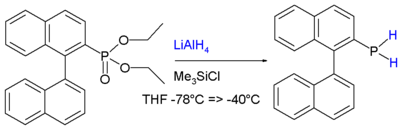 The stability is attributed to conjugation
The stability is attributed to conjugation
between the aromatic ring
and the phosphorus lone pair
.
s (R2C=PR) and phosphaalkyne
s (RC≡P). They are similar in structure, but not in reactivity, to imine
s (R2C=NR) and nitrile
s (RC≡N), respectively. In the compound phosphorine
, one carbon atom in benzene is replaced by phosphorus. Species of this type are relatively rare but for that reason are of interest to researchers. A general method for the synthesis of phosphaalkenes is by 1,2-elimination
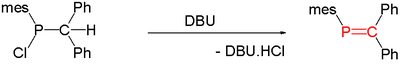
of suitable precursors, initiated thermally or by base such as DBU
, DABCO
, or triethylamine
:
Thermolysis of Me2PH generates CH2=PMe, an unstable species in the condensed phase.
. With the formulae (RP)n and (R2P)2, respectively, compounds of phosphorus(I) and (II) are generated by reduction of the related organophosphorus(III) chlorides:
Diphosphene
s, with the formula R2P2, formally contain phosphorus-phosphorus double bonds. These phosphorus(I) species are rare but are stable provided that the organic substituents are large enough to prevent catenation
. Many mixed-valence compounds are known, e.g. the cage P7(CH3)3.
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
s containing carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
–phosphorus
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
bonds
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
(thus excluding from phosphate and phosphite ester
Phosphite ester
A phosphite ester or organophosphite is a type of chemical compound with the general structure P3. Phosphite esters can be considered as esters of phosphorous acid, H3PO3. A simple phosphite ester is trimethylphosphite, P3...
s, which lack such kind of bonding), used primarily in pest control
Pest control
Pest control refers to the regulation or management of a species defined as a pest, usually because it is perceived to be detrimental to a person's health, the ecology or the economy.-History:...
as an alternative to chlorinated hydrocarbons that persist in the environment. Organophosphorus chemistry is the corresponding science of the properties and reactivity of organophosphorus compounds. Phosphorus shares group 5
Nitrogen group
The nitrogen group is a periodic table group consisting of nitrogen , phosphorus , arsenic , antimony , bismuth and ununpentium ....
in the periodic table with nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
and phosphorus compounds and nitrogen compounds are somewhat related.
The definition of organophosphorus compounds is variable, which can lead to confusion. In industrial and environmental chemistry, an organophosphorus compound need contain only an organic substituent, but need not have a direct P-C bond. Thus most pesticides, e.g., malathion
Malathion
Malathion is an organophosphate parasympathomimetic which binds irreversibly to cholinesterase. Malathion is an insecticide of relatively low human toxicity, however one recent study has shown that children with higher levels of organophosphate pesticide metabolites in their urine are more likely...
, are often included in this class of compounds.
Phosphorus can adopt a variety of oxidation state
Oxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
s, and it is general to classify organophosphorus compounds based on their being derivatives of phosphorus(V) vs phosphorus(III), which are the predominant classes of compounds. In a descriptive but only intermittently used nomenclature, phosphorus compounds are identified by their coordination number
Coordination number
In chemistry and crystallography, the coordination number of a central atom in a molecule or crystal is the number of its nearest neighbours. This number is determined somewhat differently for molecules and for crystals....
δ
Delta (letter)
Delta is the fourth letter of the Greek alphabet. In the system of Greek numerals it has a value of 4. It was derived from the Phoenician letter Dalet...
and their valency
Valence (chemistry)
In chemistry, valence, also known as valency or valence number, is a measure of the number of bonds formed by an atom of a given element. "Valence" can be defined as the number of valence bonds...
λ
Lambda
Lambda is the 11th letter of the Greek alphabet. In the system of Greek numerals lambda has a value of 30. Lambda is related to the Phoenician letter Lamed . Letters in other alphabets that stemmed from lambda include the Roman L and the Cyrillic letter El...
. In this system, a phosphine is a δ3λ3 compound.
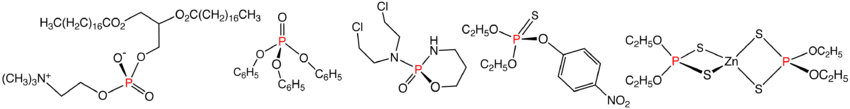
Phosphate esters and amides
Phosphate esters have the general structure P(=O)(OR)3 feature P(V). Such species are of technological importance as flame retardantFlame retardant
Flame retardants are chemicals used in thermoplastics, thermosets, textiles and coatings that inhibit or resist the spread of fire. These can be separated into several different classes of chemicals:...
agents, and plasticizer
Plasticizer
Plasticizers or dispersants are additives that increase the plasticity or fluidity of the material to which they are added; these include plastics, cement, concrete, wallboard, and clay. Although the same compounds are often used for both plastics and concretes the desired effects and results are...
s. Lacking a P−C bond, these compounds are in the technical sense not organophosphorus compounds but esters of phosphoric acid. Many derivatives are found in nature, such as phosphatidylcholine
Phosphatidylcholine
Phosphatidylcholines are a class of phospholipids that incorporate choline as a headgroup.They are a major component of biological membranes and can be easily obtained from a variety of readily available sources such as egg yolk or soy beans from which they are mechanically extracted or chemically...
. Phosphate ester are synthesized by alcoholysis of phosphorus oxychloride. A variety of mixed amido-alkoxo derivatives are known, one medically significant example being the anti-cancer drug cyclophosphamide
Cyclophosphamide
Cyclophosphamide , also known as cytophosphane, is a nitrogen mustard alkylating agent, from the oxazophorines group....
. Also derivatives containing the thiophosphoryl group (P=S) include the pesticide malathion
Malathion
Malathion is an organophosphate parasympathomimetic which binds irreversibly to cholinesterase. Malathion is an insecticide of relatively low human toxicity, however one recent study has shown that children with higher levels of organophosphate pesticide metabolites in their urine are more likely...
. The organophosphates prepared on the largest scale are the zinc dithiophosphates, as additives for motor oil. Several million kilograms of this coordination complex are produced annually by the reaction of phosphorus pentasulfide with alcohols.
In the environment, these compounds break down via hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
to eventually afford phosphate
Phosphate
A phosphate, an inorganic chemical, is a salt of phosphoric acid. In organic chemistry, a phosphate, or organophosphate, is an ester of phosphoric acid. Organic phosphates are important in biochemistry and biogeochemistry or ecology. Inorganic phosphates are mined to obtain phosphorus for use in...
and the organic alcohol or amine from which they are derived.
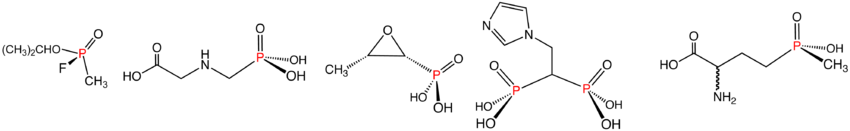
Phosphonic and phosphinic acids and their esters
PhosphonatePhosphonate
Phosphonates or phosphonic acids are organic compounds containing C-PO2 or C-PO2 groups . Bisphosphonates were first synthesized in 1897 by Von Baeyer and Hofmann. An example of such a bisphosphonate is HEDP . Since the work of Schwarzenbach in 1949, phosphonic acids are known as effective...
s are esters of phosphonic acid and have the general formula RP(=O)(OR')2. Phosphonates have many technical applications, a well-known member being Glyphosate, better known as Roundup. With the formula (HO)2P(O)CH2NHCH2CO2H, this derivative of glycine
Glycine
Glycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid...
is one of the most widely used herbicides. Bisphosphonate
Bisphosphonate
Bisphosphonates are a class of drugs that prevent the loss of bone mass, used to treat osteoporosis and similar diseases...
s are a class of drugs to treat osteoporosis
Osteoporosis
Osteoporosis is a disease of bones that leads to an increased risk of fracture. In osteoporosis the bone mineral density is reduced, bone microarchitecture is deteriorating, and the amount and variety of proteins in bone is altered...
. The nerve gas agent Sarin
Sarin
Sarin, or GB, is an organophosphorus compound with the formula [2CHO]CH3PF. It is a colorless, odorless liquid, which is used as a chemical weapon. It has been classified as a weapon of mass destruction in UN Resolution 687...
, containing both C–P and F–P bonds, is a phosphonate.
Phosphinates feature two P–C bonds, with the general formula R2P(=O)(OR'). A commercially significant member is the herbicide Glufosinate
Glufosinate
Glufosinate or its ammonium salt DL-phosphinothricin is an active ingredient in several nonselective systemic herbicides - Basta, Rely, Finale, Ignite, Challenge and Liberty. It interferes with the biosynthetic pathway of the amino acid glutamine and with ammonia detoxification.Some plants have...
. Similar to Glyphosate mentioned above, it has the structure CH3P(O)(OH)CH2CH2CH(NH2)CO2H.
The Michaelis–Arbuzov reaction is the main method for the synthesis of these compounds. For example, dimethylmethylphosphonate (see figure above) arises from the rearrangement of trimethylphosphite, which is catalyzed by methyl iodide. In the Horner–Wadsworth–Emmons reaction and the Seyferth–Gilbert homologation, phosphonates are used in reactions with carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
compounds. The Kabachnik–Fields reaction is a method for the preparation of aminophosphonates. These compounds contain a very inert bond between phosphorus and carbon. Consequently, they hydrolyze to give phosphonic and phosphinic acid derivatives, but not phosphate.
Phosphine oxides, imides, and chalcogenides
Phosphine oxides (designation δ3λ3) have the general structure R3P=O with formal oxidation state V. Phosphine oxides form hydrogen bondHydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
s and some are therefore soluble in water. The P=O bond is very polar with a dipole moment of 4.51 D for triphenylphosphine oxide
Triphenylphosphine oxide
Triphenylphosphine oxide is the chemical compound with the formula OP3. Often chemists abbreviate the formula by writing Ph3PO or PPh3O . This white crystalline compound is a common side product in reactions involving triphenylphosphine...
.
Compounds related to phosphine oxides are the imide
Imide
In organic chemistry, an imide is a functional group consisting of two carbonyl groups bound to nitrogen. These compounds are structurally related to acid anhydrides. The relationship between esters and amides and between imides and anhydrides is analogous, the amine-derived groups are less reactive...
s (R3PNR') and related chalcogenide
Chalcogenide
A chalcogenide is a chemical compound consisting of at least one chalcogen ion and at least one more electropositive element. Although all group 16 elements of the periodic table are defined as chalcogens, the term is more commonly reserved for sulfides, selenides, and tellurides, rather than...
s (R3PE, where E = S
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
, Se
Selenium
Selenium is a chemical element with atomic number 34, chemical symbol Se, and an atomic mass of 78.96. It is a nonmetal, whose properties are intermediate between those of adjacent chalcogen elements sulfur and tellurium...
, Te). These compounds are some of the most thermally stable organophosphorus compounds, but few are useful in significant amounts.
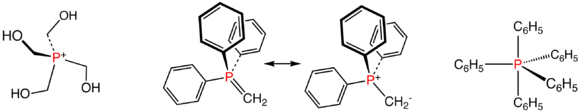
Phosphonium salts and phosphoranes
Compounds with the formula [PR4+]X- comprise the phosphonium saltsPhosphonium
The phosphonium cation describes positively charged polyatomic cations with the chemical formula . Salts of the parent PH4+ are rarely encountered, but this ion is an intermediate in the preparation of the industrially useful tetrakisphosphonium chloride:Organic phosphonium salts are common...
. These species are tetrahedral phosphorus(V) compounds. From the commercial perspective, the most important member is tetrakis(hydroxymethyl)phosphonium chloride
Tetrakis(hydroxymethyl)phosphonium chloride
Tetrakisphosphonium chloride is a phosphonium salt with the chemical formula [4P]Cl. The cation 4P+ is a four-coordinate phosphorus compound with the phosphorus atom carrying a positive charge...
, [P(CH2OH)4]Cl, which is used as a fire retardant in textile
Textile
A textile or cloth is a flexible woven material consisting of a network of natural or artificial fibres often referred to as thread or yarn. Yarn is produced by spinning raw fibres of wool, flax, cotton, or other material to produce long strands...
s. Approximately 2M kg are produced annually of the chloride and the related sulfate. They are generated by the reaction of phosphine with formaldehyde
Formaldehyde
Formaldehyde is an organic compound with the formula CH2O. It is the simplest aldehyde, hence its systematic name methanal.Formaldehyde is a colorless gas with a characteristic pungent odor. It is an important precursor to many other chemical compounds, especially for polymers...
in the presence of the mineral acid:
- PH3 + HX + 4 CH2O → [P(CH2OH)4+]X-
A variety of phosphonium salts can be prepared by alkylation
Alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion or a carbene . Alkylating agents are widely used in chemistry because the alkyl group is probably the most common group encountered in...
and arylation of organophosphines:
- PR3 + R'X → [PR3R'+]X-
The methylation of triphenylphosphine is the first step in the preparation of the Wittig reagent.
The parent phosphorane
Phosphorane
A phosphorane is a functional group in organophosphorus chemistry with pentavalent phosphorus. It has the general formula PR5. The parent hydride compound is the unstable molecule PH5...
(δ5λ5) is PH5, which is unknown. Related compounds containing both halide and organic substituents on phosphorus are fairly common. Those with five organic substituents are rare, although P(C6H5)5 is known, being derived from P(C6H5)4+ by reaction with phenyllithium
Phenyllithium
Phenyllithium is an organometallic agent with the empirical formula C6H5Li. It is most commonly used as a metalating agent in organic syntheses and a substitute for Grignard reagents for introducing phenyl groups in organic syntheses...
.
Phosphorus ylide
Ylide
An ylide or ylid is a neutral dipolar molecule containing a formally negatively charged atom directly attached to a hetero atom with a formal positive charge , and in which both atoms have full octets of electrons. Ylides are thus 1,2-dipolar compounds...
s are unsaturated phosphoranes, known as Wittig reagents, e.g. CH2P(C6H5)3. These compounds feature tetrahedral phosphorus(V) and are considered relatives of phosphine oxides. They also are derived from phosphonium salts, but by deprotonation not alkylation.
Phosphites, phosphonites, and phosphinites
Phosphites, sometimes called phosphite esterPhosphite ester
A phosphite ester or organophosphite is a type of chemical compound with the general structure P3. Phosphite esters can be considered as esters of phosphorous acid, H3PO3. A simple phosphite ester is trimethylphosphite, P3...
s, have the general structure P(OR)3 with oxidation state +3. Such species arise from the alcoholysis of phosphorus trichloride:
- PCl3 + 3 ROH → P(OR)3 + 3 HCl
The reaction is general, thus a vast number of such species are known. Phosphites are employed in the Perkow reaction
Perkow reaction
The Perkow reaction is an organic reaction in which a trialkyl phosphite ester reacts with a haloketone to form a dialkyl vinyl phosphate and an alkyl halide....
and the Michaelis–Arbuzov reaction. They also serve as ligands in organometallic chemistry.
Intermediate between phosphites and phosphines are phosphonite
Phosphonite
Phosphonites are organophosphorus compounds with the formula P2R. They are derivivatives of phosphonous acid.- See also :*Phosphine - PR3*Phosphine oxide - OPR3*Phosphinite - PR2*Phosphinate - OPR2...
s (P(OR)2R') and phosphinite
Phosphinite
Phosphinites are organophosphorus compounds with the formula PR2. They are esters of phosphinous acid.-See also:*Phosphine - PR3*Phosphine oxide - OPR3*Phosphonite - P2R*Phosphite - P3*Phosphinate - OPR2*Phosphonate - OP2R...
(P(OR)R'2). Such species arise via alcoholysis reactions of the corresponding phosphinous and phosphonous chlorides ((PClR'2) and PCl2R', respectively).
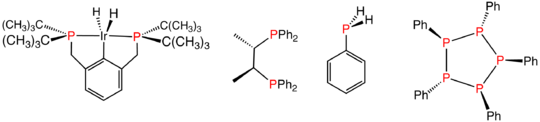
Phosphines
The parent compound of the phosphines is PH3, called phosphinePhosphine
Phosphine is the compound with the chemical formula PH3. It is a colorless, flammable, toxic gas. Pure phosphine is odourless, but technical grade samples have a highly unpleasant odor like garlic or rotting fish, due to the presence of substituted phosphine and diphosphine...
in the US and British Commonwealth, but phosphane elsewhere. Replacement of one or more hydrogen centers by an organic substituents (alkyl, aryl), gives PH3−xRx, an organophosphine, generally referred to as phosphines.
Comparison of phosphines and amines
The phosphorus atom in phosphines has a formal oxidation state −3 (δ3λ3) and are the phosphorus analogues of amineAmine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s. Like amines, phosphines have a trigonal pyramidal molecular geometry although often with smaller C-E-C angles (E = N, P), at least in the absence of steric effects. The C-P-C bond angle is 98.6° for trimethylphosphine increasing to 109.7° when the methyl groups are replaced by tert-butyl groups. When used as ligands, the steric bulk of tertiary phosphines is evaluated by their cone angle
Ligand cone angle
The ligand cone angle is a measure of the size of a ligand. It is defined as the solid angle formed with the metal at the vertex and the hydrogen atoms at the perimeter of the cone . Tertiary phosphine ligands are commonly classified using this parameter, but the method can be applied to any...
. The barrier to inversion is also much higher than in amines for a process like nitrogen inversion
Nitrogen inversion
In chemistry, a nitrogen compound like ammonia in a trigonal pyramid geometry undergoes rapid nitrogen inversion whereby the molecule turns inside out. This interconversion is a room temperature process because the energy barrier is relatively small. Contrast this to phosphine which does not show...
to occur, and therefore phosphines with three different substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
s can be resolved into thermally stable optical isomers. Phosphines are often less basic than corresponding amines, for instance the phosphonium ion itself has a pKa
PKA
PKA, pKa, or other similar variations may stand for:* pKa, the symbol for the acid dissociation constant at logarithmic scale* Protein kinase A, a class of cAMP-dependent enzymes* Pi Kappa Alpha, the North-American social fraternity...
of −14 compared to 9.21 for the ammonium ion; trimethylphosphonium
Trimethylphosphine
Trimethylphosphine is the organophosphorus compound with the formula P3, commonly abbreviated PMe3. This colorless liquid has a strongly unpleasant odour, which is characteristic of alkylphosphines. It is a pyramidal molecule with C3v symmetry, similar to ammonia and phosphine . As a ligand, its...
has a pKa
PKA
PKA, pKa, or other similar variations may stand for:* pKa, the symbol for the acid dissociation constant at logarithmic scale* Protein kinase A, a class of cAMP-dependent enzymes* Pi Kappa Alpha, the North-American social fraternity...
of 8.65 compared to 9.76 for trimethylamine
Trimethylamine
Trimethylamine is an organic compound with the formula N3. This colorless, hygroscopic, and flammable tertiary amine has a strong "fishy" odor in low concentrations and an ammonia-like odor at higher concentrations...
. However, triphenylphosphine (pKa 2.73) is more basic than triphenylamine
Triphenylamine
Triphenylamine is an organic compound with formula 3N. In contrast to most amines, triphenylamine is nonbasic. Its derivatives have useful properties in electrical conductivity and electroluminescence, and they are used in OLEDs as hole-transporters....
(pKa −5), mainly because the lone pair of the nitrogen in NPh3 is partially delocalized into the three phenyl rings. Whereas the lone pair on nitrogen is delocalized in pyrrole
Pyrrole
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4H4NH. It is a colourless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., N-methylpyrrole, C4H4NCH3...
, the lone pair on phosphorus atom in the phosphorus equivalent of pyrrole
Pyrrole
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4H4NH. It is a colourless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., N-methylpyrrole, C4H4NCH3...
(phosphole
Phosphole
Phosphole is the organic compound with the chemical formula C4H4PH; it is the phosphorus analog of pyrrole. The term phosphole also refers to substituted derivatives of the parent heterocycle...
) is not. The reactivity of phosphines matches that of amines with regard to nucleophilicity in the formation of phosphonium salt
Phosphonium salt
A phosphonium salt is a salt containing the phosphonium ion such as phosphonium iodide . More commonly, phosphonium refers to a quaternary organic derivative such as tetraphenylphosphonium chloride, 4P+ Cl- and tetramethylphosphonium iodide, [P4]+I−.Alkyltriphenylphosphonium salts are widely...
s with the general structure PR4+X−. This property is used in the Appel reaction
Appel reaction
The Appel reaction is an organic reaction that converts an alcohol into an alkyl chloride using triphenylphosphine and carbon tetrachloride. The use of carbon tetrabromide or bromine as a halide source will yield alkyl bromides, whereas using methyl iodide or iodine gives alkyl iodides...
for converting alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s to alkyl halides. Phosphines are easily oxidized to the corresponding phosphine oxide
Phosphine oxide
Phosphine oxides are either inorganic phosphorus compounds such as phosphoryl trichloride or organophosphorus compounds with the formula OPR3, where R = alkyl or aryl...
s, whereas amine oxides are less readily generated. In part for this reason, phosphines are very rarely encountered in nature.
Synthetic routes
From the commercial perspective, the most important phosphine is triphenylphosphineTriphenylphosphine
Triphenylphosphine is a common organophosphorus compound with the formula P3 - often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature...
, several million kilograms being produced annually. It is prepared from the reaction of chlorobenzene
Chlorobenzene
Chlorobenzene is an aromatic organic compound with the chemical formula C6H5Cl. This colorless, flammable liquid is a common solvent and a widely used intermediate in the manufacture of other chemicals.-Uses:...
, PCl3
Phosphorus trichloride
Phosphorus trichloride is a chemical compound of phosphorus and chlorine, having chemical formula PCl3. Its shape is trigonal pyramidal. It is the most important of the three phosphorus chlorides. It is an important industrial chemical, being used for the manufacture of organophosphorus compounds...
, and sodium. Phosphines of a more specialized nature are usually prepared by other routes. Phosphorus halides undergo Nucleophilic displacement by organometallic reagents such as Grignard reagents. Conversely, some syntheses entail nucleophilic displacement of phosphide anion equivalents ("R2P-") by aryl- and alkyl halides. Primary (RPH2) and secondary phosphines (RRPH and R2PH) add to alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s in presence of a strong base (e.g., KOH
Potassium hydroxide
Potassium hydroxide is an inorganic compound with the formula KOH, commonly called caustic potash.Along with sodium hydroxide , this colorless solid is a prototypical strong base. It has many industrial and niche applications. Most applications exploit its reactivity toward acids and its corrosive...
in DMSO
Dimethyl sulfoxide
Dimethyl sulfoxide is an organosulfur compound with the formula 2SO. This colorless liquid is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water...
). Markovnikov's rule
Markovnikov's rule
In organic chemistry, Markovnikov's rule or Markownikoff's rule is an observation based on Zaitsev's rule. It was formulated by the Russian chemist Vladimir Vasilevich Markovnikov in 1870....
s apply. Similar reactions occur involving alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
s. Base is not required for electron-deficient alkenes (e.g., derivatives of acrylonitrile
Acrylonitrile
Acrylonitrile is the chemical compound with the formula C3H3N. This pungent-smelling colorless liquid often appears yellow due to impurities. It is an important monomer for the manufacture of useful plastics. In terms of its molecular structure, it consists of a vinyl group linked to a nitrile...
) and alkynes.
Under free-radical conditions the P-H bonds of primary and secondary phosphines add across alkenes. Such reactions proceed with anti-Markovnikov regiochemistry. AIBN or organic peroxide
Peroxide
A peroxide is a compound containing an oxygen–oxygen single bond or the peroxide anion .The O−O group is called the peroxide group or peroxo group. In contrast to oxide ions, the oxygen atoms in the peroxide ion have an oxidation state of −1.The simplest stable peroxide is hydrogen peroxide...
s are used as initiator
Initiator
An initiator can refer to:* A person that takes an initiative in making something happen.* Modulated neutron initiator, a neutron source used in some nuclear weapons...
s. Tertiary phosphine oxides and sulfides can be reduced with chlorosilane
Chlorosilane
Chlorosilanes are a group of reactive, chlorine-containing chemical compounds, related to silane and used in many chemical processes. Each such chemical has at least one silicon-chlorine bond.-Synthesis:...
s and other reagents.
Reactions
The main reaction types of phosphines are as nucleophiles and bases. Their nucleophilicity is evidence by their reactions with alkyl halides to phosphonium saltPhosphonium salt
A phosphonium salt is a salt containing the phosphonium ion such as phosphonium iodide . More commonly, phosphonium refers to a quaternary organic derivative such as tetraphenylphosphonium chloride, 4P+ Cl- and tetramethylphosphonium iodide, [P4]+I−.Alkyltriphenylphosphonium salts are widely...
s. Phosphines are nucleophilic catalysts in the dimerization of enones in various reactions in organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
, e.g. the Rauhut–Currier reaction.
Phosphines are reducing agent
Reducing agent
A reducing agent is the element or compound in a reduction-oxidation reaction that donates an electron to another species; however, since the reducer loses an electron we say it is "oxidized"...
s, as illustrated in the Staudinger reduction converting azides to amines and in the Mitsunobu reaction
Mitsunobu reaction
The Mitsunobu reaction is an organic reaction that converts an alcohol into a variety of functional groups, such as an ester, using triphenylphosphine and an azodicarboxylate such as diethyl azodicarboxylate or diisopropyl azodicarboxylate . The alcohol undergoes an inversion of stereochemistry...
for converting alcohols into esters. In these processes, the phosphine is oxidized to the phosphine oxide. Phosphines have also been found to reduce activated carbonyl groups for instance the reduction of an α-keto ester to an α-hydroxy ester in scheme 2. In the proposed reaction mechanism
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
, the first proton is on loan from the methyl group in trimethylphosphine (triphenylphosphine does not react).
Phosphine ligands
Phosphines such as trimethylphosphineTrimethylphosphine
Trimethylphosphine is the organophosphorus compound with the formula P3, commonly abbreviated PMe3. This colorless liquid has a strongly unpleasant odour, which is characteristic of alkylphosphines. It is a pyramidal molecule with C3v symmetry, similar to ammonia and phosphine . As a ligand, its...
are important ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
s in inorganic chemistry. Mainly owing to the utility of asymmetric synthesis, a variety of chiral diphosphines
Diphosphines
Diphosphines, sometimes called bisphosphanes are organophosphorus compounds that are used as ligands in inorganic and organometallic chemistry. They are identified by the presence of two phosphino groups linked by a backbone and are usually chelating...
have been popularized, such as BINAP
BINAP
BINAP is an abbreviation for the organophosphorus compound 2,2'-bis-1,1'-binaphthyl. This chiral ligand is widely used in asymmetric synthesis. It consists of a pair of 2-diphenylphosphinonaphthyl groups linked at the 1 and 1´ positions. This C2-symmetric framework lacks stereogenic atom, but...
and DIPAMP
DIPAMP
DIPAMP is an organophosphorus compound that is used as a ligand in homogeneous catalysis. It is a white solid that dissolves in organic solvents. Work on this compound by W. S. Knowles was recognized with the Nobel Prize in Chemistry. DIPAMP was the basis for of the first practical asymmetric...
. A large number of phosphine ligands including diphosphines are simply called "phos ligands".
| sPhos SPhos SPhos is an organophosphorus compound derived from biphenyl. Its palladium complexes exhibit high activity for Suzuki coupling reactions involving aryl chlorides, which are unreactive with palladium complexes of most other phosphine ligands. The ligand has convenient handling characteristics since... |
 |
SPANphos SPANphos SPANphos is an organophosphorus compound used as a ligand in organometallic and coordination chemistry. This compound is a rare example of a trans-spanning ligand and rigidly links mutually trans coordination sites. By virtue of its chiral backbone that forms a chiral cavity over the face of a... |
 |
| SEGphos SEGPHOS SEGPHOS is a chiral ligand that is used in asymmetric synthesis. It was developed after BINAP and was investigated since it has a narrower dihedral angle between the aromatic faces. This was predicted and then confirmed to increase the enantioselectivity and activity of metal complexes of SEGPHOS.... |
 |
Triphos Triphos Triphos is the name for two organophosphorus compounds. Both are air sensitive white solids that serve as tridentate ligands in coordination and organometallic chemistry... |
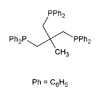 |
| Xantphos Xantphos Xantphos is an organophosphorus compound derived from the heterocycle xanthene. It is used as a bidentate ligand and is noteworthy for having a particularly wide bite angle. Such ligands are useful in the hydroformylation of alkenes. Illustrative of its wide bite angle, it forms both cis and... |
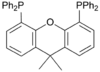 |
XPhos XPhos XPhos is an organophosphorus compound derived from biphenyl. Its palladium complexes exhibit high activity for Buchwald-Hartwig amination reactions involving aryl chlorides and aryl tosylates. Both palladium and copper complexes of the compound exhibit high activity for the coupling of aryl... |
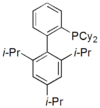 |
| Chiraphos Chiraphos Chiraphos is a chiral diphosphine employed as a ligand in organometallic chemistry. This bidentate ligand chelates metals via the two phosphine groups. Its name is derived from its description — being both chiral and a phosphine... |
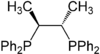 |
duPhos DuPhos DuPhos is a class of asymmetric ligands for asymmetric synthesis. The name DuPhos is derived from the chemical company that developed this type of ligand and the compound class of phospholanes it belongs to. This diphosphine ligand type was introduced in 1991 by M.J... |
|
| A selection of phos ligands | |||
Primary and secondary phosphines
In addition to the other reactions associated with phosphines, those bearing P-H groups exhibit additional reactivity associated with the P-H bonds. They are readily deprotonated using strong bases to give phosphide anions. Primary and secondary phosphines are generally prepared by reduction of related phosphorus halides or esters. For example, phosphonates are reduced to primary phosphines:
Conjugated system
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
between the aromatic ring
Aromaticity
In organic chemistry, Aromaticity is a chemical property in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. The earliest use of the term was in an article by August...
and the phosphorus lone pair
Lone pair
In chemistry, a lone pair is a valence electron pair without bonding or sharing with other atoms. They are found in the outermost electron shell of an atom, so lone pairs are a subset of a molecule's valence electrons...
.
Phosphaalkenes and phosphaalkynes
Compounds with carbon phosphorus(III) multiple bonds are called phosphaalkenePhosphaalkene
Phosphaalkenes are organophosphorus compounds with double bonds between carbon and phosphorus with the formula R2C=PR. In the compound phosphorine one carbon atom in benzene is replaced by phosphorus...
s (R2C=PR) and phosphaalkyne
Phosphaalkyne
In chemistry, phosphaalkynes are organophosphorus compounds that have a phosphorus-carbon triple bond....
s (RC≡P). They are similar in structure, but not in reactivity, to imine
Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
s (R2C=NR) and nitrile
Nitrile
A nitrile is any organic compound that has a -C≡N functional group. The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, one example being super glue .Inorganic compounds containing the -C≡N group are not called...
s (RC≡N), respectively. In the compound phosphorine
Phosphorine
Phosphorine is a heavier element analog of pyridine, containing a phosphorus atom instead of an aza- moiety. It is also called phosphabenzene and belongs to the phosphaalkene class. Phosphorine is a planar aromatic compound with 88% of the aromaticity of that of benzene...
, one carbon atom in benzene is replaced by phosphorus. Species of this type are relatively rare but for that reason are of interest to researchers. A general method for the synthesis of phosphaalkenes is by 1,2-elimination
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
of suitable precursors, initiated thermally or by base such as DBU
DBU (chemistry)
1,8-Diazabicyclo[5.4.0]undec-7-ene, or more commonly DBU, is a chemical compound and belongs to the class of amidine compounds. It is used in organic synthesis as a catalyst and complexing ligand and a strong non-nucleophilic base.It is used as a curing agent for epoxy; it is used as a protecting...
, DABCO
DABCO
DABCO or 1,4-diazabicyclo[2.2.2]octane is a chemical compound. It is a polyurethane and Baylis-Hillman reaction catalyst, complexing ligand and Lewis base. It is used to regulate the reaction rate in Flexplay time-limited DVDs by adjusting pH. Antioxidants, like DABCO, are used to improve the...
, or triethylamine
Triethylamine
Triethylamine is the chemical compound with the formula N3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine, for which TEA is also a common abbreviation....
:
Thermolysis of Me2PH generates CH2=PMe, an unstable species in the condensed phase.
Organophosphorus(0), (I), and (II) compounds
Compounds where phosphorus exists in a formal oxidation state of less than III are uncommon, but examples are known for each class. Organophosphorus(0) species are illustrated by the carbene adducts, [P(NHC)]2, where NHC is an N-heterocyclic carbenePersistent carbene
A persistent carbene is a type of carbene demonstrating particular stability. The best-known examples are diaminocarbenes with the general formula 2C:, where the 'R's are various functional groups...
. With the formulae (RP)n and (R2P)2, respectively, compounds of phosphorus(I) and (II) are generated by reduction of the related organophosphorus(III) chlorides:
- 5 PhPCl2DichlorophenylphosphineDichlorophenylphosphine is an organophosphorus compound with the formula C6H5PCl2. This colourless viscous liquid is commonly used in the synthesis of phosphine ligands....
+ 5 Mg → (PhP)5 + 5 MgCl2 - 2 Ph2PClChlorodiphenylphosphineChlorodiphenylphosphine is an organophosphorus compound with the formula 2PCl, abbreviated Ph2PCl. It is a colourless oily liquid with a pungent odor that is often described as being garlic-like and detectable even in the ppb range. It is useful reagent for introducing the Ph2P group into...
+ Mg → Ph2P-PPh2 + MgCl2
Diphosphene
Diphosphene
In chemistry, a diphosphene is an organophosphorus compound that has a phosphorus-phosphorus double bond, denoted by R-P=P-R'. These compounds are not common but are of theoretical interest. Normally, compounds with the empirical formula RP exist as rings. However, when the organic substituent is...
s, with the formula R2P2, formally contain phosphorus-phosphorus double bonds. These phosphorus(I) species are rare but are stable provided that the organic substituents are large enough to prevent catenation
Catenation
Catenation is the ability of a chemical element to form a long chain-like structure via a series of covalent bonds. Catenation occurs most readily in carbon, which forms covalent bonds with other carbon atoms. Catenation is the reason for the presence of a large number of organic compounds in nature...
. Many mixed-valence compounds are known, e.g. the cage P7(CH3)3.
See also
- Activity based proteomicsActivity based proteomicsActivity based proteomics, or activity based protein profiling is a functional proteomic technology that uses specially designed chemical probes that react with mechanistically-related classes of enzymes. The basic unit of ABPP is the probe which typically consists of two elements: a reactive...
a branch of biochemistry that often relies on organophosphorus probes to interrogate enzyme activities - Organophosphates
- OrganothiophosphatesOrganothiophosphatesOrganothiophosphates are organic compounds that include a phosphorus-sulfur bond.Many of these compounds are quite toxic, and some are used as pesticides. Examples of these include:* Chlorpyrifos* Diazinon* Fenitrothion* Fenthion* Malathion...
External links
- organophosphorus chemistry @ users.ox.ac.uk; @ www.chem.wisc.edu
- NMR predictor for organophosphorus compound chemical shifts from Alan Brisdon's Research Group Link