
Alcohol oxidation
Encyclopedia
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
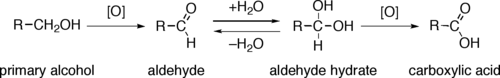
. Primary alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s (R-CH2-OH) can be oxidized either to aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s (R-CHO) or to carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
s (R-CO2H), while the oxidation of secondary alcohols (R1R2CH-OH) normally terminates at the ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
(R1R2C=O) stage. Tertiary alcohols (R1R2R3C-OH) are resistant to oxidation .
The direct oxidation of primary alcohols to carboxylic acids normally proceeds via the corresponding aldehyde, which is transformed via an aldehyde hydrate (R-CH(OH)2) by reaction with water before it can be further oxidized to the carboxylic acid.
Often it is possible to interrupt the oxidation of a primary alcohol at the aldehyde level by performing the reaction in absence of water, so that no aldehyde hydrate can be formed.
Oxidation to aldehydes
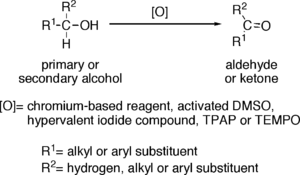
- Chromium-based reagents, such as Collins reagentCollins reagentCollins reagent is the complex of chromium oxide with pyridine in dichloromethane. It is used to selectively oxidize primary alcohols to the aldehyde, and will tolerate many other functional groups within the molecule....
(CrO3·Py2), PDC or PCCPyridinium chlorochromatePyridinium chlorochromate is a reddish orange solid reagent used to oxidize primary alcohols to aldehydes and secondary alcohols to ketones. Pyridinium chlorochromate, or PCC, will not fully oxidize a primary alcohol to the carboxylic acid as does the Jones reagent. A disadvantage to using PCC is...
. - Activated DMSODimethyl sulfoxideDimethyl sulfoxide is an organosulfur compound with the formula 2SO. This colorless liquid is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water...
, resulting from reaction of DMSO with electrophileElectrophileIn general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
s, such as oxalyl chlorideOxalyl chlorideOxalyl chloride or ethanedioyl dichloride is a chemical compound with the formula 2. This colourless, sharp-smelling liquid, the diacid chloride of oxalic acid, is a useful reagent in organic synthesis...
(Swern oxidationSwern oxidationThe Swern oxidation, named after Daniel Swern, is a chemical reaction whereby a primary or secondary alcohol is oxidized to an aldehyde or ketone using oxalyl chloride, dimethyl sulfoxide and an organic base, such as triethylamine...
), a carbodiimideCarbodiimideA carbodiimide or a methanediimine is a functional group consisting of the formula RN=C=NR. Carbodiimides hydrolyze to form ureas, which makes them uncommon in nature.-Carbodiimide formation:...
(Pfitzner-Moffatt oxidationPfitzner-Moffatt oxidationThe Pfitzner–Moffatt oxidation, sometimes referred to as simply the Moffatt oxidation, is a chemical reaction which describes the oxidation of primary and secondary alcohols by dimethyl sulfoxide activated with a carbodiimide, such as dicyclohexylcarbodiimide...
) or the complex SO3·Py (Parikh-Doering oxidationParikh-Doering oxidationThe Parikh-Doering oxidation is an oxidation reaction that transforms primary and secondary alcohols into aldehydes and ketones, respectively. The procedure uses DMSO as the oxidant, activated by sulfur trioxide-pyridine complex in the presence of triethylamine base:The procedure can be run at...
). - Hypervalent iodine compounds, such as Dess-Martin periodinaneDess-Martin periodinaneDess–Martin periodinane is a chemical reagent used to oxidize primary alcohols to aldehydes and secondary alcohols to ketones. This periodinane has several advantages over chromium- and DMSO-based oxidants that include milder conditions , shorter reaction times, higher yields, simplified workups,...
or 2-Iodoxybenzoic acid2-Iodoxybenzoic acidIBX acid or 2-iodoxybenzoic acid is an organic compound used in organic synthesis as an oxidizing agent. This Periodinane is especially suited to oxidize alcohols to aldehydes. The IBX acid is prepared from 2-iodobenzoic acid, potassium bromate and sulfuric acid...
. - Catalytic TPAP in presence of excess of NMON-Methylmorpholine N-oxideN-Methylmorpholine-N-oxide, NMO or NMMO is an organic compound. This heterocyclic amine oxide and morpholine derivative is used in organic chemistry as a co-oxidant and sacrificial catalyst in oxidation reactions for instance in osmium tetroxide oxidations and the Sharpless asymmetric...
(Ley oxidation). - Catalytic TEMPOTEMPOoxyl, or oxidanyl or TEMPO is a chemical compound with the formula 32NO . This heterocycle is a red-orange, sublimable solid. As a stable radical, it has applications throughout chemistry and biochemistry. TEMPO was discovered by Lebedev and Kazarnowskii in 1960...
in presence of excess bleachBleachBleach refers to a number of chemicals that remove color, whiten, or disinfect, often via oxidation. Common chemical bleaches include household chlorine bleach , lye, oxygen bleach , and bleaching powder...
(NaOCl) (Anelli's oxidation).
Allylic and benzylic alcohols can be oxidized in presence of other alcohols using certain selective oxidants such as manganese dioxide (MnO2).
Oxidation to ketones
Reagents useful for the oxidation of secondary alcohols to ketones, but normally inefficient for oxidation of primary alcohols to aldehydes, include chromium trioxideChromium trioxide
Chromium trioxide is the inorganic compound with the formula CrO3. It is the acidic anhydride of chromic acid, and is sometimes marketed under the same name.This compound is a dark-red/orange brown solid, which dissolves in water concomitant with hydrolysis...
(CrO3) in a mixture of sulfuric acid and acetone
Acetone
Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
(Jones oxidation
Jones oxidation
The Jones oxidation, is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones, respectively. It is named after its discoverer, Sir Ewart Jones....
) and certain ketones, such as cyclohexanone
Cyclohexanone
Cyclohexanone is the organic compound with the formula 5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oil has an odor reminiscent of peardrop sweets as well as acetone. Over time, samples assume a yellow color due to oxidation...
, in the presence of aluminium isopropoxide
Aluminium isopropoxide
Aluminium isopropoxide is the chemical compound usually described with the formula Al3, where i-Pr is the isopropyl group . This colourless solid is a useful reagent in organic synthesis...
(Oppenauer oxidation
Oppenauer oxidation
Oppenauer oxidation, named after Rupert Viktor Oppenauer, is a gentle method for selectively oxidizing secondary alcohols to ketones.The reaction is the opposite of Meerwein-Ponndorf-Verley reduction. The alcohol is oxidized with aluminium isopropoxide in excess acetone...
). Another method is oxoammonium-catalyzed oxidation
Oxoammonium-catalyzed oxidation
Oxoammonium-catalyzed oxidation reactions involve the conversion of organic substrates to more highly oxidized materials through the action of an N-oxoammonium species. Nitroxides may also be used in catalytic amounts in the presence of a stoichiometric amount of a terminal...
.
Oxidation to carboxylic acids

The direct oxidation of primary alcohols to carboxylic acids can be carried out using:
- Potassium permanganatePotassium permanganatePotassium permanganate is an inorganic chemical compound with the formula KMnO4. It is a salt consisting of K+ and MnO4− ions. Formerly known as permanganate of potash or Condy's crystals, it is a strong oxidizing agent. It dissolves in water to give intensely purple solutions, the...
(KMnO4). - Jones oxidationJones oxidationThe Jones oxidation, is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones, respectively. It is named after its discoverer, Sir Ewart Jones....
. - PDC in DMF2,5-Dimethylfuran2,5-Dimethylfuran is a heterocyclic compound with the formula 2C4H2O. Although often abbreviated DMF, it should not be confused with dimethylformamide. A derivative of furan, this simple compound is a potential biofuel, being derivable from cellulose.-Production:Fructose can be converted into...
. - Heyns oxidation.
- Ruthenium tetroxideRuthenium tetroxideRuthenium tetroxide is a diamagnetic tetrahedral ruthenium compound. As expected for a charge-neutral symmetrical oxide, it is quite volatile. The analogous OsO4 is more widely used and better known...
(RuO4). - TEMPOTEMPOoxyl, or oxidanyl or TEMPO is a chemical compound with the formula 32NO . This heterocycle is a red-orange, sublimable solid. As a stable radical, it has applications throughout chemistry and biochemistry. TEMPO was discovered by Lebedev and Kazarnowskii in 1960...
.
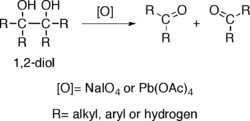
Diol oxidation
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
groups located on adjacent carbons —that is, 1,2-diol
Diol
A diol or glycol is a chemical compound containing two hydroxyl groups A geminal diol has two hydroxyl groups bonded to the same atom...
s— suffer oxidative breakage at a carbon-carbon bond with some oxidants such as sodium periodate
Sodium periodate
Sodium periodate is the sodium salt of periodic acid. It can refer to two different chemical compounds, sodium metaperiodate , which has the formula NaIO4, and sodium orthoperiodate , which has the formula Na2H3IO6...
(NaIO4) or lead tetraacetate (Pb(OAc)4), resulting in generation of two carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
groups. The reaction is also known as glycol cleavage
Glycol cleavage
Glycol cleavage is a specific type of organic chemistry oxidation. The carbon–carbon bond in a Glycol cleavage is a specific type of organic chemistry oxidation. The carbon–carbon bond in a Glycol cleavage is a specific type of organic chemistry oxidation. The carbon–carbon bond in a (vicinal diol...
.

