Resistivity
Encyclopedia
Electrical resistivity is a measure of how strongly a material opposes the flow of electric current
. A low resistivity indicates a material that readily allows the movement of electric charge
. The SI
unit of electrical resistivity is the ohm
metre
(Ωm). It is commonly represented by the Greek letter
ρ
(rho).
Electrical conductivity or specific conductance is the reciprocal quantity, and measures a material's ability to conduct an electric current
. It is commonly represented by the Greek letter σ (sigma), but κ
(esp. in electrical engineering) or γ
are also occasionally used. Its SI unit is siemens
per metre
(S·m−1) and CGSE unit
is reciprocal second
(s−1):
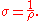
 Electrical resistivity ρ (Greek: rho
Electrical resistivity ρ (Greek: rho
) is defined by,
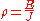
where
and E and J are both inside the conductor.
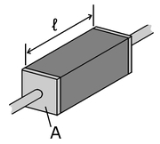
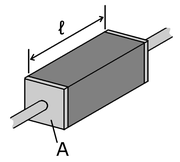
Many resistor
s and conductors
have a uniform cross section with a uniform flow of electric current and are made of one material. (See the diagram to the right.) In this case, the above definition of ρ leads to:
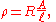
where
The reason resistivity has the dimension units of ohm-metres can be seen by transposing the definition to make resistance the subject:
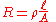
The resistance of a given sample will increase with the length, but decrease with greater cross-sectional area. Resistance is measured in ohms. Length over area has units of 1/distance. To end up with ohms, resistivity must be in the units of "ohms × distance" (SI ohm-metre, US ohm-inch).
In a hydraulic analogy
, increasing the cross-sectional area of a pipe reduces its resistance to flow, and increasing the length increases resistance to flow (and pressure drop for a given flow).
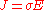
or equivalently
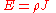
is only valid for resistors and conductors which are homogeneous and isotropic, that is the conductivity is uniform throughout the conductor, carrying a uniform electric current per unit cross-sectional area J, through a uniform internal electric field E, all in one dimension only.
For all three dimensions we have at all points (r = position vector at a point in the conductor):

in which (1, 2, 3 refer to components)

This is still only true if E and J are collinear, under the same conditions as above.
Since E and J are generally vector fields, the material may not be homogeneous or isotropic so the conductivity may vary from point to point, and the E and J are not always collinear at the same point, the definition generalizes by treating conductivity as a rank-2 tensor field
σ:

where rank-2 tensors can be represented by matrices
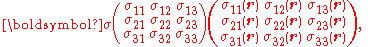
equivalent to the matrix equation
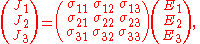
which allows a simple relation between components to be read off, using the Einstein summation convention;
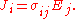
The above tensor equation serves as a definition for the conductivity tensor (or field). A similar analysis for resistivity can be done, introducing a resistivity tensor (or field) in an identical form,
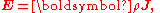
in which
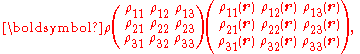
so
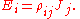
The degree of doping
in semiconductors makes a large difference in conductivity. To a point, more doping leads to higher conductivity. The conductivity of a solution of water is highly dependent on its concentration
of dissolved salts, and other chemical species that ionize
in the solution. Electrical conductivity of water samples is used as an indicator of how salt-free, ion-free, or impurity-free the sample is; the purer the water, the lower the conductivity (the higher the resistivity). Conductivity measurements in water are often reported as specific conductance, relative to the conductivity of pure water at 25 °C. An EC meter
is normally used to measure conductivity in a solution.
This table shows the resistivity, conductivity and temperature coefficient
of various materials at 20 °C
(68 °F
)
The effective temperature coefficient varies with temperature and purity level of the material. The 20 °C value is only an approximation when used at other temperatures. For example, the coefficient becomes lower at higher temperatures for copper, and the value 0.00427 is commonly specified at 0 °C. For further reading: http://library.bldrdoc.gov/docs/nbshb100.pdf.
The extremely low resistivity (high conductivity) of silver is characteristic of metals. George Gamow
tidily summed up the nature of the metals' dealings with electrons in his science-popularizing book, One, Two, Three...Infinity (1947): "The metallic substances differ from all other materials by the fact that the outer shells of their atoms are bound rather loosely, and often let one of their electrons go free. Thus the interior of a metal is filled up with a large number of unattached electrons that travel aimlessly around like a crowd of displaced persons. When a metal wire is subjected to electric force applied on its opposite ends, these free electrons rush in the direction of the force, thus forming what we call an electric current." More technically, the free electron model
gives a basic description of electron flow in metals.
s increases with temperature
, while the resistivity of intrinsic semiconductor
s decreases with increasing temperature. In both cases, electron–phonon
interactions can play a key role. At high temperatures, the resistance of a metal increases linearly with temperature. As the temperature of a metal is reduced, the temperature dependence of resistivity follows a power law function of temperature. Mathematically the temperature dependence of the resistivity ρ of a metal is given by the Bloch–Grüneisen formula:
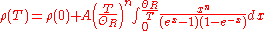
where is the residual resistivity due to defect scattering, A is a constant that depends on the velocity of electrons at the Fermi surface
is the residual resistivity due to defect scattering, A is a constant that depends on the velocity of electrons at the Fermi surface
, the Debye radius and the number density of electrons in the metal.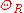 is the Debye temperature as obtained from resistivity measurements and matches very closely with the values of Debye temperature obtained from specific heat measurements. n is an integer that depends upon the nature of interaction:
is the Debye temperature as obtained from resistivity measurements and matches very closely with the values of Debye temperature obtained from specific heat measurements. n is an integer that depends upon the nature of interaction:
As the temperature of the metal is sufficiently reduced (so as to 'freeze' all the phonons), the resistivity usually reaches a
constant value, known as the residual resistivity. This value depends not only on the type of metal, but on its purity and thermal history. The value of the residual resistivity of a metal is decided by its impurity concentration. Some materials lose all electrical resistivity at sufficiently low temperatures, due to an effect known as superconductivity
.
An even better approximation of the temperature dependence of the resistivity of a semiconductor is given by the Steinhart–Hart equation:
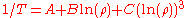
where A, B and C are the so-called Steinhart–Hart coefficients.
This equation is used to calibrate thermistor
s.
In non-crystalline semi-conductors, conduction can occur by charges quantum tunnelling
from one localised site to another. This is known as variable range hopping and has the characteristic form of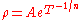 , where n=2,3,4 depending on the dimensionality of the system.
, where n=2,3,4 depending on the dimensionality of the system.
s, in applications such as electrical impedance tomography
, it is necessary to replace resistivity with a complex
quantity called impeditivity (in analogy to electrical impedance
). Impeditivity is the sum of a real component, the resistivity, and an imaginary component, the reactivity (in analogy to reactance). The magnitude of Impeditivity is the square root of sum of squares of magnitudes of resistivity and reactivity.
Conversely, in such cases the conductivity must be expressed as a complex number
(or even as a matrix of complex numbers, in the case of anisotropic materials) called the admittivity
. Admittivity is the sum of a real component called the conductivity and an imaginary component called the susceptivity
.
An alternative description of the response to alternating currents uses a real (but frequency-dependent) conductivity, along with a real permittivity
. The larger the conductivity is, the more quickly the alternating-current signal is absorbed by the material (i.e., the more opaque
the material is). For details, see Mathematical descriptions of opacity
.
s— aluminium is frequently used rather than copper because it is lighter for the same conductance.
Silver, although it is the least resistive metal known, has a high density and does poorly by this measure. Calcium and the alkali metals make for the best products, but are rarely used for conductors due to their high reactivity with water and oxygen. Aluminium is far more stable. And the most important attribute, the current price, excludes the best choice: Beryllium.
Electric current
Electric current is a flow of electric charge through a medium.This charge is typically carried by moving electrons in a conductor such as wire...
. A low resistivity indicates a material that readily allows the movement of electric charge
Electric charge
Electric charge is a physical property of matter that causes it to experience a force when near other electrically charged matter. Electric charge comes in two types, called positive and negative. Two positively charged substances, or objects, experience a mutual repulsive force, as do two...
. The SI
Si
Si, si, or SI may refer to :- Measurement, mathematics and science :* International System of Units , the modern international standard version of the metric system...
unit of electrical resistivity is the ohm
Ohm
The ohm is the SI unit of electrical resistance, named after German physicist Georg Simon Ohm.- Definition :The ohm is defined as a resistance between two points of a conductor when a constant potential difference of 1 volt, applied to these points, produces in the conductor a current of 1 ampere,...
metre
Metre
The metre , symbol m, is the base unit of length in the International System of Units . Originally intended to be one ten-millionth of the distance from the Earth's equator to the North Pole , its definition has been periodically refined to reflect growing knowledge of metrology...
(Ωm). It is commonly represented by the Greek letter
Greek alphabet
The Greek alphabet is the script that has been used to write the Greek language since at least 730 BC . The alphabet in its classical and modern form consists of 24 letters ordered in sequence from alpha to omega...
ρ
Rho (letter)
Rho is the 17th letter of the Greek alphabet. In the system of Greek numerals, it has a value of 100. It is derived from Semitic resh "head"...
(rho).
Electrical conductivity or specific conductance is the reciprocal quantity, and measures a material's ability to conduct an electric current
Electric current
Electric current is a flow of electric charge through a medium.This charge is typically carried by moving electrons in a conductor such as wire...
. It is commonly represented by the Greek letter σ (sigma), but κ
Kappa
Kappa is the 10th letter of the Greek alphabet, used to represent the voiceless velar stop, or "k", sound in Ancient and Modern Greek. In the system of Greek numerals it has a value of 20. It was derived from the Phoenician letter Kaph...
(esp. in electrical engineering) or γ
Gamma
Gamma is the third letter of the Greek alphabet. In the system of Greek numerals it has a value of 3. It was derived from the Phoenician letter Gimel . Letters that arose from Gamma include the Roman C and G and the Cyrillic letters Ge Г and Ghe Ґ.-Greek:In Ancient Greek, gamma represented a...
are also occasionally used. Its SI unit is siemens
Siemens (unit)
The siemens is the SI derived unit of electric conductance and electric admittance. Conductance and admittance are the reciprocals of resistance and impedance respectively, hence one siemens is equal to the reciprocal of one ohm, and is sometimes referred to as the mho. In English, the term...
per metre
Metre
The metre , symbol m, is the base unit of length in the International System of Units . Originally intended to be one ten-millionth of the distance from the Earth's equator to the North Pole , its definition has been periodically refined to reflect growing knowledge of metrology...
(S·m−1) and CGSE unit
Electrostatic units
The electrostatic system of units is a system of units used to measure electrical quantities of electric charge, current, and voltage, within the centimeter gram second metric system of units. In electrostatic units, electrical charge is defined via the force it exerts on other charges...
is reciprocal second
Second
The second is a unit of measurement of time, and is the International System of Units base unit of time. It may be measured using a clock....
(s−1):
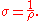
Scalar form

Rho (letter)
Rho is the 17th letter of the Greek alphabet. In the system of Greek numerals, it has a value of 100. It is derived from Semitic resh "head"...
) is defined by,
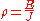
where
- ρ is the static resistivity (measured in ohm-metres, Ω-m)
- E is the magnitudeMagnitude (mathematics)The magnitude of an object in mathematics is its size: a property by which it can be compared as larger or smaller than other objects of the same kind; in technical terms, an ordering of the class of objects to which it belongs....
of the electric fieldElectric fieldIn physics, an electric field surrounds electrically charged particles and time-varying magnetic fields. The electric field depicts the force exerted on other electrically charged objects by the electrically charged particle the field is surrounding...
(measured in voltVoltThe volt is the SI derived unit for electric potential, electric potential difference, and electromotive force. The volt is named in honor of the Italian physicist Alessandro Volta , who invented the voltaic pile, possibly the first chemical battery.- Definition :A single volt is defined as the...
s per metreMetreThe metre , symbol m, is the base unit of length in the International System of Units . Originally intended to be one ten-millionth of the distance from the Earth's equator to the North Pole , its definition has been periodically refined to reflect growing knowledge of metrology...
, V/m), - J is the magnitude of the current densityCurrent densityCurrent density is a measure of the density of flow of a conserved charge. Usually the charge is the electric charge, in which case the associated current density is the electric current per unit area of cross section, but the term current density can also be applied to other conserved...
(measured in ampereAmpereThe ampere , often shortened to amp, is the SI unit of electric current and is one of the seven SI base units. It is named after André-Marie Ampère , French mathematician and physicist, considered the father of electrodynamics...
s per square metreSquare metreThe square metre or square meter is the SI derived unit of area, with symbol m2 . It is defined as the area of a square whose sides measure exactly one metre...
, A/m²),
and E and J are both inside the conductor.
Many resistor
Resistor
A linear resistor is a linear, passive two-terminal electrical component that implements electrical resistance as a circuit element.The current through a resistor is in direct proportion to the voltage across the resistor's terminals. Thus, the ratio of the voltage applied across a resistor's...
s and conductors
Electrical conductor
In physics and electrical engineering, a conductor is a material which contains movable electric charges. In metallic conductors such as copper or aluminum, the movable charged particles are electrons...
have a uniform cross section with a uniform flow of electric current and are made of one material. (See the diagram to the right.) In this case, the above definition of ρ leads to:
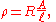
where
- R is the electrical resistanceElectrical resistanceThe electrical resistance of an electrical element is the opposition to the passage of an electric current through that element; the inverse quantity is electrical conductance, the ease at which an electric current passes. Electrical resistance shares some conceptual parallels with the mechanical...
of a uniform specimen of the material (measured in ohmOhmThe ohm is the SI unit of electrical resistance, named after German physicist Georg Simon Ohm.- Definition :The ohm is defined as a resistance between two points of a conductor when a constant potential difference of 1 volt, applied to these points, produces in the conductor a current of 1 ampere,...
s, Ω)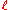 is the length of the piece of material (measured in metreMetreThe metre , symbol m, is the base unit of length in the International System of Units . Originally intended to be one ten-millionth of the distance from the Earth's equator to the North Pole , its definition has been periodically refined to reflect growing knowledge of metrology...
is the length of the piece of material (measured in metreMetreThe metre , symbol m, is the base unit of length in the International System of Units . Originally intended to be one ten-millionth of the distance from the Earth's equator to the North Pole , its definition has been periodically refined to reflect growing knowledge of metrology...
s, m) - A is the cross-sectional area of the specimen (measured in square metres, m²).
The reason resistivity has the dimension units of ohm-metres can be seen by transposing the definition to make resistance the subject:
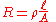
The resistance of a given sample will increase with the length, but decrease with greater cross-sectional area. Resistance is measured in ohms. Length over area has units of 1/distance. To end up with ohms, resistivity must be in the units of "ohms × distance" (SI ohm-metre, US ohm-inch).
In a hydraulic analogy
Hydraulic analogy
The electronic–hydraulic analogy is the most widely used analogy for "electron fluid" in a metal conductor. Since electric current is invisible and the processes at play in electronics are often difficult to demonstrate, the various electronic components are represented by hydraulic...
, increasing the cross-sectional area of a pipe reduces its resistance to flow, and increasing the length increases resistance to flow (and pressure drop for a given flow).
Tensor generalization
The above scalar equation: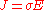
or equivalently
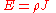
is only valid for resistors and conductors which are homogeneous and isotropic, that is the conductivity is uniform throughout the conductor, carrying a uniform electric current per unit cross-sectional area J, through a uniform internal electric field E, all in one dimension only.
For all three dimensions we have at all points (r = position vector at a point in the conductor):

in which (1, 2, 3 refer to components)

This is still only true if E and J are collinear, under the same conditions as above.
Since E and J are generally vector fields, the material may not be homogeneous or isotropic so the conductivity may vary from point to point, and the E and J are not always collinear at the same point, the definition generalizes by treating conductivity as a rank-2 tensor field
Tensor field
In mathematics, physics and engineering, a tensor field assigns a tensor to each point of a mathematical space . Tensor fields are used in differential geometry, algebraic geometry, general relativity, in the analysis of stress and strain in materials, and in numerous applications in the physical...
σ:

where rank-2 tensors can be represented by matrices
Matrix (mathematics)
In mathematics, a matrix is a rectangular array of numbers, symbols, or expressions. The individual items in a matrix are called its elements or entries. An example of a matrix with six elements isMatrices of the same size can be added or subtracted element by element...
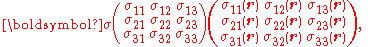
equivalent to the matrix equation
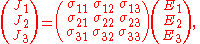
which allows a simple relation between components to be read off, using the Einstein summation convention;
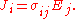
The above tensor equation serves as a definition for the conductivity tensor (or field). A similar analysis for resistivity can be done, introducing a resistivity tensor (or field) in an identical form,
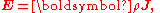
in which
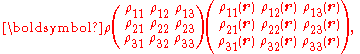
so
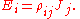
Resistivity of various materials
- A conductorElectrical conductorIn physics and electrical engineering, a conductor is a material which contains movable electric charges. In metallic conductors such as copper or aluminum, the movable charged particles are electrons...
such as a metalMetalA metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
has high conductivity and a low resistivity. - An insulatorElectrical insulationthumb|250px|[[Coaxial Cable]] with dielectric insulator supporting a central coreThis article refers to electrical insulation. For insulation of heat, see Thermal insulation...
like glassGlassGlass is an amorphous solid material. Glasses are typically brittle and optically transparent.The most familiar type of glass, used for centuries in windows and drinking vessels, is soda-lime glass, composed of about 75% silica plus Na2O, CaO, and several minor additives...
has low conductivity and a high resistivity. - The conductivity of a semiconductorSemiconductorA semiconductor is a material with electrical conductivity due to electron flow intermediate in magnitude between that of a conductor and an insulator. This means a conductivity roughly in the range of 103 to 10−8 siemens per centimeter...
is generally intermediate, but varies widely under different conditions, such as exposure of the material to electric fields or specific frequencies of lightLightLight or visible light is electromagnetic radiation that is visible to the human eye, and is responsible for the sense of sight. Visible light has wavelength in a range from about 380 nanometres to about 740 nm, with a frequency range of about 405 THz to 790 THz...
, and, most important, with temperatureTemperatureTemperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
and composition of the semiconductor material.
The degree of doping
Doping (semiconductor)
In semiconductor production, doping intentionally introduces impurities into an extremely pure semiconductor for the purpose of modulating its electrical properties. The impurities are dependent upon the type of semiconductor. Lightly and moderately doped semiconductors are referred to as extrinsic...
in semiconductors makes a large difference in conductivity. To a point, more doping leads to higher conductivity. The conductivity of a solution of water is highly dependent on its concentration
Concentration
In chemistry, concentration is defined as the abundance of a constituent divided by the total volume of a mixture. Four types can be distinguished: mass concentration, molar concentration, number concentration, and volume concentration...
of dissolved salts, and other chemical species that ionize
Ionization
Ionization is the process of converting an atom or molecule into an ion by adding or removing charged particles such as electrons or other ions. This is often confused with dissociation. A substance may dissociate without necessarily producing ions. As an example, the molecules of table sugar...
in the solution. Electrical conductivity of water samples is used as an indicator of how salt-free, ion-free, or impurity-free the sample is; the purer the water, the lower the conductivity (the higher the resistivity). Conductivity measurements in water are often reported as specific conductance, relative to the conductivity of pure water at 25 °C. An EC meter
EC meter
An electrical conductivity meter measures the electrical conductivity in a solution. Commonly used in hydroponics, aquaculture and freshwater systems to monitor the amount of nutrients, salts or impurities in the water....
is normally used to measure conductivity in a solution.
This table shows the resistivity, conductivity and temperature coefficient
Temperature coefficient
The temperature coefficient is the relative change of a physical property when the temperature is changed by 1 K.In the following formula, let R be the physical property to be measured and T be the temperature at which the property is measured. T0 is the reference temperature, and ΔT is the...
of various materials at 20 °C
Celsius
Celsius is a scale and unit of measurement for temperature. It is named after the Swedish astronomer Anders Celsius , who developed a similar temperature scale two years before his death...
(68 °F
Fahrenheit
Fahrenheit is the temperature scale proposed in 1724 by, and named after, the German physicist Daniel Gabriel Fahrenheit . Within this scale, the freezing of water into ice is defined at 32 degrees, while the boiling point of water is defined to be 212 degrees...
)
| Material | ρ [Ω·m] at | σ [S/m] at | Temperature coefficientThe numbers in this column increase or decrease the significand Significand The significand is part of a floating-point number, consisting of its significant digits. Depending on the interpretation of the exponent, the significand may represent an integer or a fraction.-Examples:... portion of the resistivity. For example, at 30 °C (303.2 K), the resistivity of silver is 1.65×10−8. This is calculated as Δρ = α ΔT ρo where ρo is the resistivity at 20 °C (in this case) and α is the temperature coefficient. [K−1] |
Reference |
|---|---|---|---|---|
| Silver Silver Silver is a metallic chemical element with the chemical symbol Ag and atomic number 47. A soft, white, lustrous transition metal, it has the highest electrical conductivity of any element and the highest thermal conductivity of any metal... |
1.59×10-8 | 6.30×107 | 0.0038 | |
| Copper Copper Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish... |
1.68×10-8 | 5.96×107 | 0.0039 | |
| Annealed Annealing (metallurgy) Annealing, in metallurgy and materials science, is a heat treatment wherein a material is altered, causing changes in its properties such as strength and hardness. It is a process that produces conditions by heating to above the recrystallization temperature, maintaining a suitable temperature, and... Copper Copper Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish... Referred to as 100% IACS or International Annealed Copper Standard. The unit for expressing the conductivity of nonmagnetic materials by testing using the eddy-current method. Generally used for temper and alloy verification of aluminium. |
1.72×10-8 | 5.80×107 | ||
| Gold Gold Gold is a chemical element with the symbol Au and an atomic number of 79. Gold is a dense, soft, shiny, malleable and ductile metal. Pure gold has a bright yellow color and luster traditionally considered attractive, which it maintains without oxidizing in air or water. Chemically, gold is a... Gold is commonly used in electrical contacts because it does not easily corrode. |
2.44×10-8 | |4.10×107 | 0.0034 | |
| Aluminium Aluminium Aluminium or aluminum is a silvery white member of the boron group of chemical elements. It has the symbol Al, and its atomic number is 13. It is not soluble in water under normal circumstances.... Commonly used for high voltage power lines |
2.82×10-8 | 3.5×107 | 0.0039 | |
| Calcium Calcium Calcium is the chemical element with the symbol Ca and atomic number 20. It has an atomic mass of 40.078 amu. Calcium is a soft gray alkaline earth metal, and is the fifth-most-abundant element by mass in the Earth's crust... |
3.36×10-8 | 2.98×107 | 0.0041 | |
| Tungsten Tungsten Tungsten , also known as wolfram , is a chemical element with the chemical symbol W and atomic number 74.A hard, rare metal under standard conditions when uncombined, tungsten is found naturally on Earth only in chemical compounds. It was identified as a new element in 1781, and first isolated as... |
5.60×10-8 | 1.79×107 | 0.0045 | |
| Zinc Zinc Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2... |
5.90×10-8 | 1.69×107 | 0.0037 | |
| Nickel Nickel Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile... |
6.99×10-8 | 1.43×107 | 0.006 | |
| Lithium Lithium Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly... |
9.28×10-8 | 1.08×107 | 0.006 | |
| Iron Iron Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust... |
1.0×10-7 | 1.00×107 | 0.005 | |
| Platinum Platinum Platinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal... |
1.06×10-7 | 9.43×106 | 0.00392 | |
| Tin Tin Tin is a chemical element with the symbol Sn and atomic number 50. It is a main group metal in group 14 of the periodic table. Tin shows chemical similarity to both neighboring group 14 elements, germanium and lead and has two possible oxidation states, +2 and the slightly more stable +4... |
1.09×10-7 | 9.17×106 | 0.0045 | |
| Lead Lead Lead is a main-group element in the carbon group with the symbol Pb and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed... |
2.2×10-7 | 4.55×106 | 0.0039 | |
| Titanium Titanium Titanium is a chemical element with the symbol Ti and atomic number 22. It has a low density and is a strong, lustrous, corrosion-resistant transition metal with a silver color.... |
4.20×10-7 | 2.38×106 | X | |
| Manganin Manganin Manganin is a trademarked name for an alloy of typically 86% copper, 12% manganese, and 2% nickel. It was first developed by Edward Weston in 1892, improving upon his Constantan .... |
4.82×10-7 | 2.07×106 | 0.000002 | |
| Constantan Constantan Constantan is a copper-nickel alloy usually consisting of 55% copper and 45% nickel.Also known as Eureka.Its main feature is its resistivity which is constant over a wide range of temperatures... |
4.9×10-7 | 2.04×106 | 0.000008 | |
| Stainless steel Stainless steel In metallurgy, stainless steel, also known as inox steel or inox from French "inoxydable", is defined as a steel alloy with a minimum of 10.5 or 11% chromium content by mass.... 18% chromium/ 8% nickel austenitic stainles steel |
6.897×10-7 | 1.450×106 | ||
| Mercury Mercury (element) Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum... |
9.8×10-7 | 1.02×106 | 0.0009 | |
| Nichrome Nichrome Nichrome is a non-magnetic alloy of nickel, chromium, and often iron, usually used as a resistance wire. Patented in 1905, it is the oldest documented form of resistance heating alloy. A common alloy is 80% nickel and 20% chromium, by mass, but there are many others to accommodate various... Nickel-Iron-Chromium alloy commonly used in heating elements. |
1.10×10-6 | 9.09×105 | 0.0004 | |
| Carbon (amorphous) Amorphous carbon Amorphous carbon or free, reactive carbon, is an allotrope of carbon that does not have any crystalline structure. As with all glassy materials, some short-range order can be observed... |
5×10-4 to 8×10-4 | 1.25 to 2×103 | −0.0005 | |
| Carbon (graphite) Graphite The mineral graphite is one of the allotropes of carbon. It was named by Abraham Gottlob Werner in 1789 from the Ancient Greek γράφω , "to draw/write", for its use in pencils, where it is commonly called lead . Unlike diamond , graphite is an electrical conductor, a semimetal... Graphite is strongly anisotropic. |
2.5e×10-6 to 5.0×10-6 ⊥basal plane 3.0×10−3 //basal plane |
2 to 3×105 ⊥basal plane 3.3×102 //basal plane |
||
| Carbon (diamond) Diamond In mineralogy, diamond is an allotrope of carbon, where the carbon atoms are arranged in a variation of the face-centered cubic crystal structure called a diamond lattice. Diamond is less stable than graphite, but the conversion rate from diamond to graphite is negligible at ambient conditions... |
1×1012 | ~10-13 | ||
| Germanium Germanium Germanium is a chemical element with the symbol Ge and atomic number 32. It is a lustrous, hard, grayish-white metalloid in the carbon group, chemically similar to its group neighbors tin and silicon. The isolated element is a semiconductor, with an appearance most similar to elemental silicon.... |
4.6×10-1 | 2.17 | −0.048 | |
| Sea waterCorresponds to an average salinity of 35 g/kg at 20 °C. | 2×10-1 | 4.8 | ||
| Drinking water Drinking water Drinking water or potable water is water pure enough to be consumed or used with low risk of immediate or long term harm. In most developed countries, the water supplied to households, commerce and industry is all of drinking water standard, even though only a very small proportion is actually... This value range is typical of high quality drinking water and not an indicator of water quality |
2×101 to 2×103 | 5×10-4 to 5×10-2 | ||
| Deionized waterConductivity is lowest with monoatomic gases present; changes to 1.2 × 10-4 upon complete de-gassing, or to 7.5×10-5 upon equilibration to the atmosphere due to dissolved CO2 | 1.8×105 | 5.5 × 10−6 | ||
| Silicon Silicon Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table... |
6.40×102 | 1.56×10-3 | −0.075 | |
| GaAs Gaas Gaas is a commune in the Landes department in Aquitaine in south-western France.... |
5×10-7 to 10×10-3 | 5×10-8 to 103 | ||
| Glass Glass Glass is an amorphous solid material. Glasses are typically brittle and optically transparent.The most familiar type of glass, used for centuries in windows and drinking vessels, is soda-lime glass, composed of about 75% silica plus Na2O, CaO, and several minor additives... |
10×1010 to 10×1014 | 10-11 to 10-15 | ? | |
| Hard rubber | 1×1013 | 10-14 | ? | |
| Sulfur Sulfur Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow... |
1×1015 | 10-16 | ? | |
| Air | 1.3×1016 to 3.3×1016 | 3 to 8 × 10−15 | ||
| Paraffin Paraffin In chemistry, paraffin is a term that can be used synonymously with "alkane", indicating hydrocarbons with the general formula CnH2n+2. Paraffin wax refers to a mixture of alkanes that falls within the 20 ≤ n ≤ 40 range; they are found in the solid state at room temperature and begin to enter the... |
1×1017 | 10-18 | ? | |
| Quartz Quartz Quartz is the second-most-abundant mineral in the Earth's continental crust, after feldspar. It is made up of a continuous framework of SiO4 silicon–oxygen tetrahedra, with each oxygen being shared between two tetrahedra, giving an overall formula SiO2. There are many different varieties of quartz,... (fused) |
7.5×1017 | 1.3×10-18 | ? | |
| PET Polyethylene terephthalate Polyethylene terephthalate , commonly abbreviated PET, PETE, or the obsolete PETP or PET-P, is a thermoplastic polymer resin of the polyester family and is used in synthetic fibers; beverage, food and other liquid containers; thermoforming applications; and engineering resins often in combination... |
10×1020 | 10-21 | ? | |
| Teflon | 10×1022 to 10×1024 | 10-25 to 10-23 | ? |
The effective temperature coefficient varies with temperature and purity level of the material. The 20 °C value is only an approximation when used at other temperatures. For example, the coefficient becomes lower at higher temperatures for copper, and the value 0.00427 is commonly specified at 0 °C. For further reading: http://library.bldrdoc.gov/docs/nbshb100.pdf.
The extremely low resistivity (high conductivity) of silver is characteristic of metals. George Gamow
George Gamow
George Gamow , born Georgiy Antonovich Gamov , was a Russian-born theoretical physicist and cosmologist. He discovered alpha decay via quantum tunneling and worked on radioactive decay of the atomic nucleus, star formation, stellar nucleosynthesis, Big Bang nucleosynthesis, cosmic microwave...
tidily summed up the nature of the metals' dealings with electrons in his science-popularizing book, One, Two, Three...Infinity (1947): "The metallic substances differ from all other materials by the fact that the outer shells of their atoms are bound rather loosely, and often let one of their electrons go free. Thus the interior of a metal is filled up with a large number of unattached electrons that travel aimlessly around like a crowd of displaced persons. When a metal wire is subjected to electric force applied on its opposite ends, these free electrons rush in the direction of the force, thus forming what we call an electric current." More technically, the free electron model
Free electron model
In solid-state physics, the free electron model is a simple model for the behaviour of valence electrons in a crystal structure of a metallic solid. It was developed principally by Arnold Sommerfeld who combined the classical Drude model with quantum mechanical Fermi-Dirac statistics and hence it...
gives a basic description of electron flow in metals.
Temperature dependence
In general, electrical resistivity of metalMetal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
s increases with temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
, while the resistivity of intrinsic semiconductor
Semiconductor
A semiconductor is a material with electrical conductivity due to electron flow intermediate in magnitude between that of a conductor and an insulator. This means a conductivity roughly in the range of 103 to 10−8 siemens per centimeter...
s decreases with increasing temperature. In both cases, electron–phonon
Phonon
In physics, a phonon is a collective excitation in a periodic, elastic arrangement of atoms or molecules in condensed matter, such as solids and some liquids...
interactions can play a key role. At high temperatures, the resistance of a metal increases linearly with temperature. As the temperature of a metal is reduced, the temperature dependence of resistivity follows a power law function of temperature. Mathematically the temperature dependence of the resistivity ρ of a metal is given by the Bloch–Grüneisen formula:
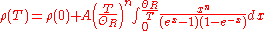
where
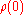 is the residual resistivity due to defect scattering, A is a constant that depends on the velocity of electrons at the Fermi surface
is the residual resistivity due to defect scattering, A is a constant that depends on the velocity of electrons at the Fermi surfaceFermi surface
In condensed matter physics, the Fermi surface is an abstract boundary useful for predicting the thermal, electrical, magnetic, and optical properties of metals, semimetals, and doped semiconductors. The shape of the Fermi surface is derived from the periodicity and symmetry of the crystalline...
, the Debye radius and the number density of electrons in the metal.
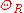 is the Debye temperature as obtained from resistivity measurements and matches very closely with the values of Debye temperature obtained from specific heat measurements. n is an integer that depends upon the nature of interaction:
is the Debye temperature as obtained from resistivity measurements and matches very closely with the values of Debye temperature obtained from specific heat measurements. n is an integer that depends upon the nature of interaction:- n=5 implies that the resistance is due to scattering of electrons by phononPhononIn physics, a phonon is a collective excitation in a periodic, elastic arrangement of atoms or molecules in condensed matter, such as solids and some liquids...
s (as it is for simple metals) - n=3 implies that the resistance is due to s-d electron scattering (as is the case for transition metals)
- n=2 implies that the resistance is due to electron–electron interaction.
As the temperature of the metal is sufficiently reduced (so as to 'freeze' all the phonons), the resistivity usually reaches a
constant value, known as the residual resistivity. This value depends not only on the type of metal, but on its purity and thermal history. The value of the residual resistivity of a metal is decided by its impurity concentration. Some materials lose all electrical resistivity at sufficiently low temperatures, due to an effect known as superconductivity
Superconductivity
Superconductivity is a phenomenon of exactly zero electrical resistance occurring in certain materials below a characteristic temperature. It was discovered by Heike Kamerlingh Onnes on April 8, 1911 in Leiden. Like ferromagnetism and atomic spectral lines, superconductivity is a quantum...
.
An even better approximation of the temperature dependence of the resistivity of a semiconductor is given by the Steinhart–Hart equation:
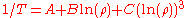
where A, B and C are the so-called Steinhart–Hart coefficients.
This equation is used to calibrate thermistor
Thermistor
A thermistor is a type of resistor whose resistance varies significantly with temperature, more so than in standard resistors. The word is a portmanteau of thermal and resistor...
s.
In non-crystalline semi-conductors, conduction can occur by charges quantum tunnelling
Quantum tunnelling
Quantum tunnelling refers to the quantum mechanical phenomenon where a particle tunnels through a barrier that it classically could not surmount. This plays an essential role in several physical phenomena, such as the nuclear fusion that occurs in main sequence stars like the sun, and has important...
from one localised site to another. This is known as variable range hopping and has the characteristic form of
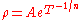 , where n=2,3,4 depending on the dimensionality of the system.
, where n=2,3,4 depending on the dimensionality of the system.Complex resistivity and conductivity
When analyzing the response of materials to alternating electric fieldElectric field
In physics, an electric field surrounds electrically charged particles and time-varying magnetic fields. The electric field depicts the force exerted on other electrically charged objects by the electrically charged particle the field is surrounding...
s, in applications such as electrical impedance tomography
Electrical impedance tomography
Electrical impedance tomography is a medical imaging technique in which an image of the conductivity or permittivity of part of the body is inferred from surface electrical measurements. Typically, conducting electrodes are attached to the skin of the subject and small alternating currents are...
, it is necessary to replace resistivity with a complex
Complex number
A complex number is a number consisting of a real part and an imaginary part. Complex numbers extend the idea of the one-dimensional number line to the two-dimensional complex plane by using the number line for the real part and adding a vertical axis to plot the imaginary part...
quantity called impeditivity (in analogy to electrical impedance
Electrical impedance
Electrical impedance, or simply impedance, is the measure of the opposition that an electrical circuit presents to the passage of a current when a voltage is applied. In quantitative terms, it is the complex ratio of the voltage to the current in an alternating current circuit...
). Impeditivity is the sum of a real component, the resistivity, and an imaginary component, the reactivity (in analogy to reactance). The magnitude of Impeditivity is the square root of sum of squares of magnitudes of resistivity and reactivity.
Conversely, in such cases the conductivity must be expressed as a complex number
Complex number
A complex number is a number consisting of a real part and an imaginary part. Complex numbers extend the idea of the one-dimensional number line to the two-dimensional complex plane by using the number line for the real part and adding a vertical axis to plot the imaginary part...
(or even as a matrix of complex numbers, in the case of anisotropic materials) called the admittivity
Admittance
In electrical engineering, the admittance is a measure of how easily a circuit or device will allow a current to flow. It is defined as the inverse of the impedance . The SI unit of admittance is the siemens...
. Admittivity is the sum of a real component called the conductivity and an imaginary component called the susceptivity
Susceptance
In electrical engineering, susceptance is the imaginary part of admittance. The inverse of admittance is impedance and the real part of admittance is conductance. In SI units, susceptance is measured in siemens...
.
An alternative description of the response to alternating currents uses a real (but frequency-dependent) conductivity, along with a real permittivity
Permittivity
In electromagnetism, absolute permittivity is the measure of the resistance that is encountered when forming an electric field in a medium. In other words, permittivity is a measure of how an electric field affects, and is affected by, a dielectric medium. The permittivity of a medium describes how...
. The larger the conductivity is, the more quickly the alternating-current signal is absorbed by the material (i.e., the more opaque
Opacity (optics)
Opacity is the measure of impenetrability to electromagnetic or other kinds of radiation, especially visible light. In radiative transfer, it describes the absorption and scattering of radiation in a medium, such as a plasma, dielectric, shielding material, glass, etc...
the material is). For details, see Mathematical descriptions of opacity
Mathematical descriptions of opacity
When an electromagnetic wave travels through a medium in which it gets absorbed , it undergoes exponential decay as described by the Beer–Lambert law. However, there are many possible ways to characterize the wave and how quickly it is absorbed...
.
Resistivity density products
In some applications where the weight of an item is very important resistivity density products are more important than absolute low resistivity- it is often possible to make the conductor thicker to make up for a higher resistivity; and then a low resistivity density product material (or equivalently a high conductance to density ratio) is desirable. For example, for long distance overhead power linePower Line
Power Line is an American political blog, providing news and commentary from a conservative point-of-view. It was originally written by three lawyers who attended Dartmouth College together: John H. Hinderaker, Scott W. Johnson, and Paul Mirengoff...
s— aluminium is frequently used rather than copper because it is lighter for the same conductance.
| Material | Resistivity [nΩ·m] | Density [g/cm³] | Resistivity-density product [nΩ·m·g/cm³] |
|---|---|---|---|
| Sodium Sodium Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride... |
47.7 | 0.97 | 46 |
| Lithium Lithium Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly... |
92.8 | 0.53 | 49 |
| Calcium Calcium Calcium is the chemical element with the symbol Ca and atomic number 20. It has an atomic mass of 40.078 amu. Calcium is a soft gray alkaline earth metal, and is the fifth-most-abundant element by mass in the Earth's crust... |
33.6 | 1.55 | 52 |
| Potassium Potassium Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are... |
72.0 | 0.89 | 64 |
| Beryllium Beryllium Beryllium is the chemical element with the symbol Be and atomic number 4. It is a divalent element which occurs naturally only in combination with other elements in minerals. Notable gemstones which contain beryllium include beryl and chrysoberyl... |
35.6 | 1.85 | 66 |
| Aluminium Aluminium Aluminium or aluminum is a silvery white member of the boron group of chemical elements. It has the symbol Al, and its atomic number is 13. It is not soluble in water under normal circumstances.... |
26.50 | 2.70 | 72 |
| Magnesium Magnesium Magnesium is a chemical element with the symbol Mg, atomic number 12, and common oxidation number +2. It is an alkaline earth metal and the eighth most abundant element in the Earth's crust and ninth in the known universe as a whole... |
43.90 | 1.74 | 76.3 |
| Copper Copper Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish... |
16.78 | 8.96 | 150 |
| Silver Silver Silver is a metallic chemical element with the chemical symbol Ag and atomic number 47. A soft, white, lustrous transition metal, it has the highest electrical conductivity of any element and the highest thermal conductivity of any metal... |
15.87 | 10.49 | 166 |
| Gold Gold Gold is a chemical element with the symbol Au and an atomic number of 79. Gold is a dense, soft, shiny, malleable and ductile metal. Pure gold has a bright yellow color and luster traditionally considered attractive, which it maintains without oxidizing in air or water. Chemically, gold is a... |
22.14 | 19.30 | 427 |
| Iron Iron Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust... |
96.1 | 7.874 | 757 |
Silver, although it is the least resistive metal known, has a high density and does poorly by this measure. Calcium and the alkali metals make for the best products, but are rarely used for conductors due to their high reactivity with water and oxygen. Aluminium is far more stable. And the most important attribute, the current price, excludes the best choice: Beryllium.
See also
- Conductivity near the percolation thresholdConductivity near the percolation thresholdIn a mixture between a dielectric and a metallic component, the conductivity \sigma and the dielectric constant \epsilon of this mixture show a critical behavior if the fraction of the metallic component reaches the percolation threshold...
- Electric effective resistance
- Electrical resistivities of the elements (data page)Electrical resistivities of the elements (data page)-Electrical resistivity:-WEL:As quoted at http://www.webelements.com/ from these sources:* G.W.C. Kaye and T. H. Laby in Tables of physical and chemical constants, Longman, London, UK, 15th edition, 1993....
- Electrical resistivity imaging
- Ohm's lawOhm's lawOhm's law states that the current through a conductor between two points is directly proportional to the potential difference across the two points...
- Sheet resistanceSheet resistanceSheet resistance is a measure of resistance of thin films that are namely uniform in thickness. It is commonly used to characterize materials made by semiconductor doping, metal deposition, resistive paste printing, and glass coating. Examples of these processes are: doped semiconductor regions...
- SI electromagnetism units
- Skin depth
- SuperconductivitySuperconductivitySuperconductivity is a phenomenon of exactly zero electrical resistance occurring in certain materials below a characteristic temperature. It was discovered by Heike Kamerlingh Onnes on April 8, 1911 in Leiden. Like ferromagnetism and atomic spectral lines, superconductivity is a quantum...

