
Mukaiyama Taxol total synthesis
Encyclopedia
Tokyo University of Science
Tokyo University of Science is a private university of science and technology in Japan...
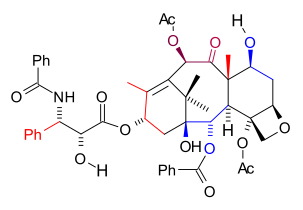
between 1997 and 1999 was the 6th successful taxol total synthesis
Taxol total synthesis
Paclitaxel total synthesis in organic chemistry is a major ongoing research effort in the total synthesis of paclitaxel . This diterpenoid is an important drug in the treatment of cancer but also expensive because the compound is harvested from a scarce resource, namely the Pacific yew...
. The total synthesis
Total synthesis
In organic chemistry, a total synthesis is, in principle, the complete chemical synthesis of complex organic molecules from simpler pieces, usually without the aid of biological processes. In practice, these simpler pieces are commercially available in bulk and semi-bulk quantities, and are often...
of Taxol is considered a hallmark in organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
.
This version is a linear synthesis with ring formation taking place in the order B, C, A, D. Contrary to the other published methods, the tail synthesis is by an own design. Teruaki Mukaiyama is an expert on aldol reaction
Aldol reaction
The aldol reaction is a powerful means of forming carbon–carbon bonds in organic chemistry.Discovered independently by Charles-Adolphe Wurtz and Alexander Porfyrevich Borodin in 1872, the reaction combines two carbonyl compounds to form a new β-hydroxy carbonyl compound...
s and not surprisingly his Taxol version contains no less than 5 of these reactions. Other key reactions encountered in this synthesis are a pinacol coupling and a Reformatskii reaction
Reformatskii reaction
The Reformatsky reaction is an organic reaction which condenses aldehydes , 1, with α-halo esters, 2, using a metallic zinc to form β-hydroxy-esters, 3...
. In terms of raw materials the C20 framework is built up from L-serine (C3), isobutyric acid
Isobutyric acid
Isobutyric acid, also known as 2-methylpropanoic acid, is a carboxylic acid with structural formula 2-CH-COOH. It is found in the free state in carobs and in the root of Arnica dulcis, and as an ethyl ester in croton oil....
(C4), glycolic acid
Glycolic acid
Glycolic acid is the smallest α-hydroxy acid . This colorless, odorless, and hygroscopic crystalline solid is highly soluble in water. It is used in various skin-care products. Glycolic acid is found in some sugar-crops...
(C2), methyl bromide (C1), methyl iodide (C1), 2,3-dibromopropene (C3), acetic acid
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
(C2) and homoallyl bromide (C4).
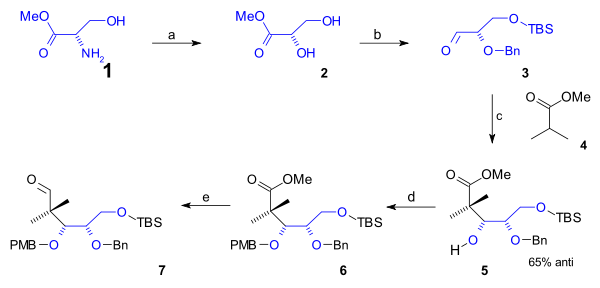
Synthesis B ring
The lower rim of the cyclooctaneCyclooctane
Cyclooctane is a cycloalkane with the molecular formula 8. It is a simple colourless hydrocarbon, but it is often a reference compound for saturated eight-membered ring compounds in general.- Conformation :...
B ring containing the first 5 carbon atoms was synthesized in a semisynthesis starting from naturally occurring L-serine (scheme 1). This route started with conversion of the amino group of the serine methyl ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
(1) to the diol
Diol
A diol or glycol is a chemical compound containing two hydroxyl groups A geminal diol has two hydroxyl groups bonded to the same atom...
ester 2 via diazotization (sodium nitrite
Sodium nitrite
Sodium nitrite is the inorganic compound with the chemical formula NaNO2. It is a white to slight yellowish crystalline powder that is very soluble in water and is hygroscopic...
/sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
). After protection of the primary alcohol group to a (t-buyldimethyl) TBS silyl ether
Silyl ether
Silyl ethers are a group of chemical compounds which contain a silicon atom covalently bonded to an alkoxy group. The general structure is R1R2R3Si−O−R4 where R4 is an alkyl group or an aryl group. Silyl ethers are usually used as protecting groups for alcohols in organic synthesis...
(TBSCl / imidazole
Imidazole
Imidazole is an organic compound with the formula C3H4N2. This aromatic heterocyclic is a diazole and is classified as an alkaloid. Imidazole refers to the parent compound, whereas imidazoles are a class of heterocycles with similar ring structure, but varying substituents...
) and that of the secondary alcohol group with a (Bn) benzyl ether (benzyl imidate, triflic acid), the aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
3 was reacted with the methyl ester of isobutyric acid
Isobutyric acid
Isobutyric acid, also known as 2-methylpropanoic acid, is a carboxylic acid with structural formula 2-CH-COOH. It is found in the free state in carobs and in the root of Arnica dulcis, and as an ethyl ester in croton oil....
(4) in an Aldol addition to alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
5 with 65% stereoselectivity
Stereoselectivity
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during the non-stereospecific creation of a new stereocenter or during the non-stereospecific transformation of a pre-existing one...
. This group was protected as a PMB (p-methoxybenzyl) ether (again through an imidate) in 6 which enabled organic reduction of the ester to the aldehyde in 7 with DIBAL.
 |
| Scheme 1 |
|---|
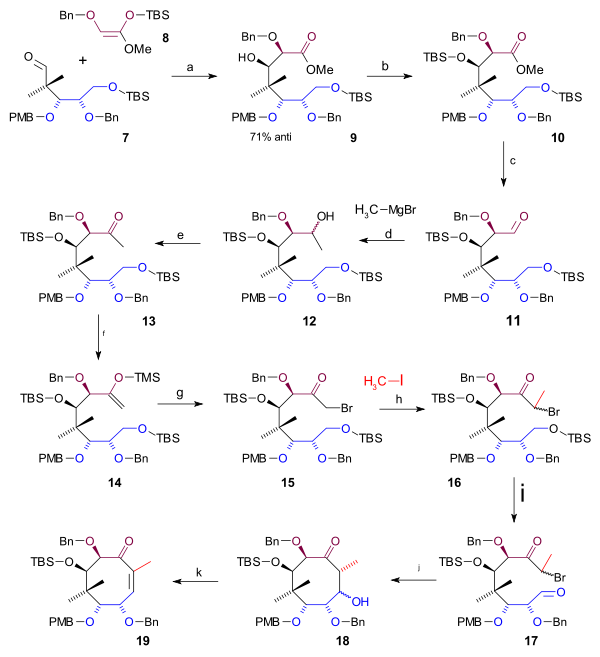
Completing the cyclooctane ring required 3 more carbon atoms that were supplied by a C2 fragment in an aldol addition and a Grignard C1 fragment (scheme 2). A Mukaiyama aldol addition
Mukaiyama aldol addition
The Mukaiyama aldol addition is an organic reaction and a type of aldol reaction between a silyl enol ether and an aldehyde catalyzed by a Lewis acid. This choice of reactants allows for a crossed aldol reaction between an aldehyde and a ketone or a different aldehyde without self-condensation of...
(magnesium bromide
Magnesium bromide
Magnesium bromide is a chemical compound of magnesium and bromine that is white and deliquescent. It is often used as a mild sedative and as an anticonvulsant for treatment of nervous disorders. It is water soluble and somewhat soluble in alcohol...
/ toluene
Toluene
Toluene, formerly known as toluol, is a clear, water-insoluble liquid with the typical smell of paint thinners. It is a mono-substituted benzene derivative, i.e., one in which a single hydrogen atom from the benzene molecule has been replaced by a univalent group, in this case CH3.It is an aromatic...
) took place between aldehyde 7 and ketene silyl acetal 8 with 71% stereoselectivity to alcohol 9 which was protected as the TBS ether 10 (TBSOTf, 2,6-lutidine
2,6-Lutidine
2,6-Lutidine is a natural heterocyclic aromatic organic compound. It has been isolated from the basic fraction of coal tar and from bone oil. It is a dimethyl substituted derivative of pyridine. It has been detected in waste water from oil shale processing sites and former creosoting facilities...
). The ester group was reduced with DIBAL to an alcohol and then back oxidized to aldehyde 11 by Swern oxidation
Swern oxidation
The Swern oxidation, named after Daniel Swern, is a chemical reaction whereby a primary or secondary alcohol is oxidized to an aldehyde or ketone using oxalyl chloride, dimethyl sulfoxide and an organic base, such as triethylamine...
. Alkylation by methyl magnesium bromide to alcohol 12 and another Swern oxidation gave ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
13. This group was converted to the silyl enol ether
Silyl enol ether
Silyl enol ethers in organic chemistry are a class of organic compounds that share a common functional group composed of an enolate bonded through its oxygen terminus to an organosilicon group....
14 (LHMDS, TMSCl) enabling it to react with NBS
N-Bromosuccinimide
N-Bromosuccinimide or NBS is a chemical reagent which is used in radical substitution and electrophilic addition reactions in organic chemistry. NBS can be considered a convenient source of cationic bromine.-Preparation:...
to alkyl bromide 15. The C20 methyl group was introduced as methyl iodide in a nucleophilic substitution
Nucleophilic substitution
In organic and inorganic chemistry, nucleophilic substitution is a fundamental class of reactions in which an electron nucleophile selectively bonds with or attacks the positive or partially positive charge of an atom or a group of atoms called the leaving group; the positive or partially positive...
with a strong base (LHMDS in HMPA) to bromide 16. Then in preparation to ring-closure the TBS ether was deprotected (HCl
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
/THF
Tetrahydrofuran
Tetrahydrofuran is a colorless, water-miscible organic liquid with low viscosity at standard temperature and pressure. This heterocyclic compound has the chemical formula 4O. As one of the most polar ethers with a wide liquid range, it is a useful solvent. Its main use, however, is as a precursor...
) to an alcohol which was converted to the aldehyde 17 in a Swern oxidation
Swern oxidation
The Swern oxidation, named after Daniel Swern, is a chemical reaction whereby a primary or secondary alcohol is oxidized to an aldehyde or ketone using oxalyl chloride, dimethyl sulfoxide and an organic base, such as triethylamine...
. The ring-closing reaction was a Reformatskii reaction
Reformatskii reaction
The Reformatsky reaction is an organic reaction which condenses aldehydes , 1, with α-halo esters, 2, using a metallic zinc to form β-hydroxy-esters, 3...
with Samarium(II) iodide
Samarium(II) iodide
Samarium iodide is a green solid composed of samarium and iodine, with a melting point of 520 °C where the samarium atom has a coordination number of seven in a capped octahedral configuration...
and acetic acid
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
to acetate
Acetate
An acetate is a derivative of acetic acid. This term includes salts and esters, as well as the anion found in solution. Most of the approximately 5 billion kilograms of acetic acid produced annually in industry are used in the production of acetates, which usually take the form of polymers. In...
18. The stereochemistry of this particular step was of no consequence because the acetate group is dehydrated
Dehydration reaction
In chemistry and the biological sciences, a dehydration reaction is usually defined as a chemical reaction that involves the loss of water from the reacting molecule. Dehydration reactions are a subset of elimination reactions...
to the alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
19 with DBU in benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
.
 |
| Scheme 2 |
|---|
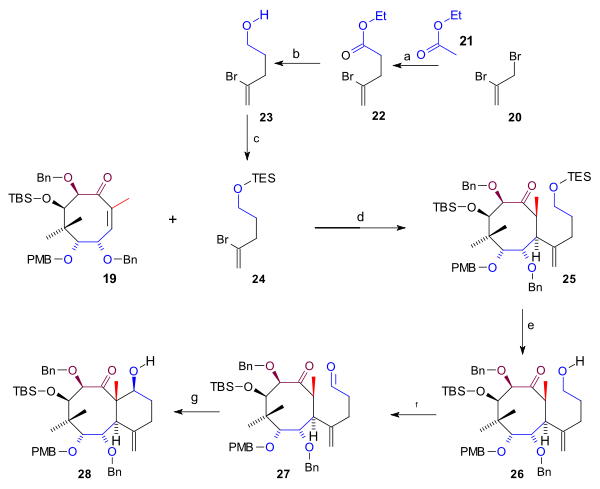
Synthesis C ring
The C5 fragment 24 required for the synthesis of the C ring (scheme 3) was prepared from 2,3-dibromopropene (20) by reaction with ethyl acetateEthyl acetate
Ethyl acetate is the organic compound with the formula CH3COOCH2CH3. This colorless liquid has a characteristic sweet smell and is used in glues, nail polish removers, and cigarettes...
(21), n-butyllithium
N-Butyllithium
n-Butyllithium is an organolithium reagent. It is widely used as a polymerization initiator in the production of elastomers such as polybutadiene or styrene-butadiene-styrene...
and a copper salt, followed by organic reduction of acetate 22 to alcohol 23 (lithium aluminium hydride
Lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH or known as LithAl, is an inorganic compound with the chemical formula LiAlH4. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters,...
) and its TES silylation
Silylation
Silylation is the introduction of a substituted silyl group to a molecule.Nearly all functional groups which present a problem in gas chromatogaphic separation can be derivatized by silylation reagents...
. Michael addition of 24 with the cyclooctane 19 to 25 with t-BuLi
Organolithium reagent
An organolithium reagent is an organometallic compound with a direct bond between a carbon and a lithium atom. As the electropositive nature of lithium puts most of the charge density of the bond on the carbon atom, effectively creating a carbanion, organolithium compounds are extremely powerful...
was catalyzed by copper cyanide. After removal of the TES group (HCl, THF), the alcohol 26 was oxidized to aldehyde 27 (TPAP, NMO
N-Methylmorpholine N-oxide
N-Methylmorpholine-N-oxide, NMO or NMMO is an organic compound. This heterocyclic amine oxide and morpholine derivative is used in organic chemistry as a co-oxidant and sacrificial catalyst in oxidation reactions for instance in osmium tetroxide oxidations and the Sharpless asymmetric...
)which enabled the intramolecular
Intramolecular
Intramolecular in chemistry describes a process or characteristic limited within the structure of a single molecule, a property or phenomenon limited to the extent of a single molecule.- Examples :...
Aldol reaction
Aldol reaction
The aldol reaction is a powerful means of forming carbon–carbon bonds in organic chemistry.Discovered independently by Charles-Adolphe Wurtz and Alexander Porfyrevich Borodin in 1872, the reaction combines two carbonyl compounds to form a new β-hydroxy carbonyl compound...
to bicycle 28.
 |
| Scheme 3 |
|---|
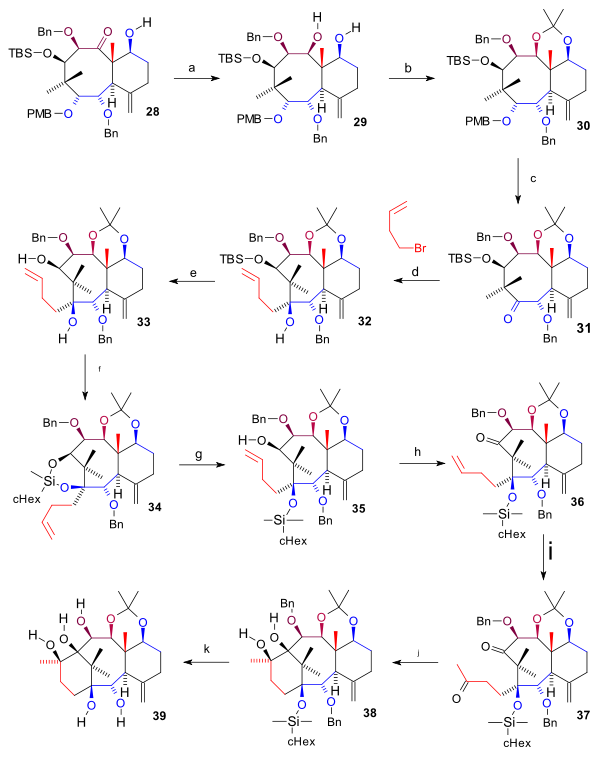
Synthesis A ring
Ring A synthesis (scheme 4) started with reduction of the C9 ketoneKetone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
group in 28 to diol
Diol
A diol or glycol is a chemical compound containing two hydroxyl groups A geminal diol has two hydroxyl groups bonded to the same atom...
29 with alane
Aluminium hydride
Aluminium hydride is an inorganic compound with the formula AlH3. It is a colourless solid that is pyrophoric. Although rarely encountered outside of research laboratory, alane and its derivatives are used as reducing agents in organic synthesis.-Structure:...
in toluene
Toluene
Toluene, formerly known as toluol, is a clear, water-insoluble liquid with the typical smell of paint thinners. It is a mono-substituted benzene derivative, i.e., one in which a single hydrogen atom from the benzene molecule has been replaced by a univalent group, in this case CH3.It is an aromatic...
followed by diol protection in 30 as a dimethyl carbonate
Carbonate ester
A carbonate ester is a functional group in organic chemistry consisting of a carbonyl group flanked by two alkoxy groups. The general structure of these carbonates is R1OOR2 and they are related to esters R1OR and ethers R1OR2 and also to the inorganic carbonates.Carbonate esters are used as...
. This allowed selective oxidation of the C1 alcohol with DDQ
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone is the chemical reagent with formula C8Cl2N2O2. This oxidant is useful for the dehydrogenation of alcohols, phenols and steroid ketones in organic chemistry. DDQ decomposes in water, but is stable in aqueous mineral acid.-Preparation:Synthesis of DDQ...
after deprotection to ketone 31. This compound was alkylated to 32 at the C1 ketone group with the Grignard homoallyl magnesium bromide (C4 fragment completing the carbon framework) and deprotected at C11 (TBAF) to diol 33. By reaction with cyclohexylmethylsilyldichloride both alcohol groups participated in a cyclic silyl ether (34) which was again cleaved by reaction with methyl lithium
Methyl lithium
Methyllithium is an organolithium reagent with the empirical formula CH3Li. This s-block organometallic compound adopts an oligomeric structure both in solution and in the solid state. This highly reactive compound, invariably used as a solution in ethers, is a reagent in organic synthesis as well...
exposing the C11 alcohol in 35. The A ring closure required two ketone groups for a pinacol coupling which were realized by oxidation of the C11 alcohol (TPAP, NMO) to ketone 36 and Wacker oxidation of the allyl group to diketone 37. After formation of the pinacol product 38 the benzyl groups (sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
, ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
) and the trialkylsilyl groups (TBAF) were removed to form pentaol 39.
 |
| Scheme 4 |
|---|
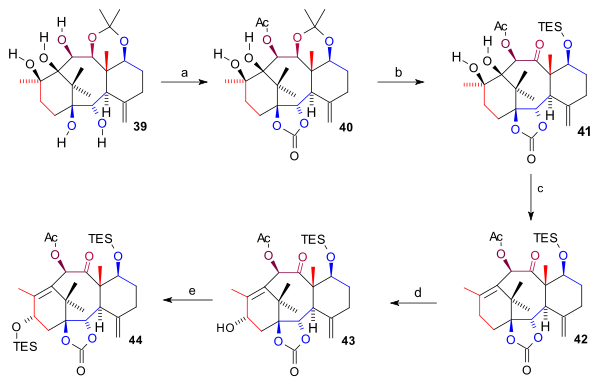
The pentaol 39 was protected twice: two bottom hydroxyl groups as a carbonate ester
Carbonate ester
A carbonate ester is a functional group in organic chemistry consisting of a carbonyl group flanked by two alkoxy groups. The general structure of these carbonates is R1OOR2 and they are related to esters R1OR and ethers R1OR2 and also to the inorganic carbonates.Carbonate esters are used as...
(bis(trichloromethyl)carbonate, pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
) and the C10 hydroxyl group as the acetate
Acetate
An acetate is a derivative of acetic acid. This term includes salts and esters, as well as the anion found in solution. Most of the approximately 5 billion kilograms of acetic acid produced annually in industry are used in the production of acetates, which usually take the form of polymers. In...
forming 40. The acetonide group was removed (HCl, THF), the C7 hydroxyl group protected as a TES silyl ether and the C11 OH group oxidized (TPAP, NMO) to ketone 41. The ring A diol group was next removed in a combined elimination reaction
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
and Barton deoxygenation with 1,1'-thiocarbonyldiimidazole forming alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
42. Finally the C15 hydroxyl group was introduced by oxidation at the allyl
Allyl
An allyl group is a substituent with the structural formula H2C=CH-CH2R, where R is the connection to the rest of the molecule. It is made up of a methylene , attached to a vinyl group . The name is derived from the Latin word for garlic, Allium sativum. Theodor Wertheim isolated an allyl...
position with in two steps PPC
4-Phenyl-4-(1-piperidinyl)cyclohexanol
4-Phenyl-4--cyclohexanol, also known as PPC, is an organic chemical which is a metabolite of phencyclidine . It can be detected in the hair of PCP users.PPC has been shown to cause increases in locomotor activity in lab mice....
and sodium acetate
Sodium acetate
Sodium acetate, CH3COONa, also abbreviated NaOAc, also sodium ethanoate, is the sodium salt of acetic acid. This colourless salt has a wide range of uses.-Industrial:...
(to the enone
Enone
An enone is an unsaturated chemical compound or functional group consisting of a conjugated system of an alkene and a ketone. The simplest enone is methyl vinyl ketone or CH2=CHCOCH3....
) and with K-selectride to alcohol 43 which was protected as a TES ether in 44.
 |
| Scheme 5 |
|---|
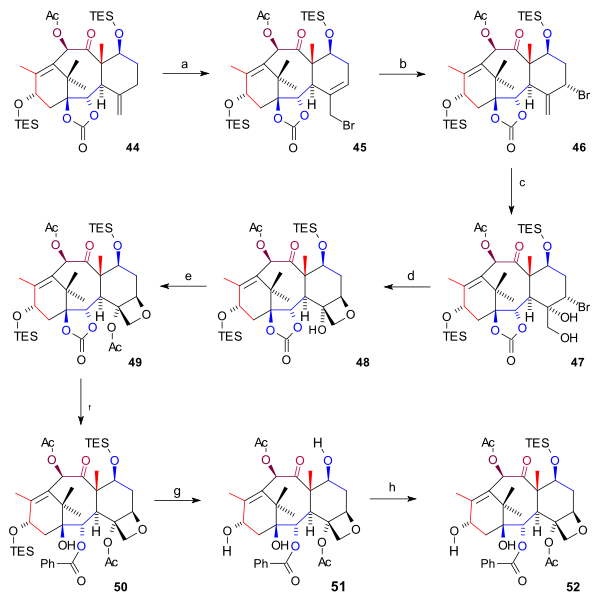
Synthesis D ring
The synthesis of the D ring (scheme 6) started from 44 with allylic bromination with copper(I) bromideCopper(I) bromide
Copper bromide is the chemical compound with the formula CuBr. This diamagnetic solid adopts a polymeric structure akin to that for zinc sulfide. The compound is widely used in the synthesis of organic compounds....
and benzoyl tert-butyl peroxide
Organic peroxide
Organic peroxides are organic compounds containing the peroxide functional group . If the R' is hydrogen, the compound is called an organic hydroperoxide. Peresters have general structure RCOOR. The O-O bond easily breaks and forms free radicals of the form RO·...
to bromide 45. By adding even more bromide, another bromide 46 formed (both compounds are in chemical equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
) with the bromine atom in an axial
Cyclohexane conformation
A cyclohexane conformation is any of several three-dimensional shapes that a cyclohexane molecule can assume while maintaining the integrity of its chemical bonds....
position. Osmium tetroxide added two hydroxyl groups to the exocyclic double bond in diol 47 and oxetane
Oxetane
Oxetane, or 1,3-propylene oxide, is an heterocyclic organic compound with the molecular formula C3H6O, having a four-membered ring with three carbon atoms and one oxygen atom....
ring-closure to 48 took place with DBU in a nucleophilic substitution
Nucleophilic substitution
In organic and inorganic chemistry, nucleophilic substitution is a fundamental class of reactions in which an electron nucleophile selectively bonds with or attacks the positive or partially positive charge of an atom or a group of atoms called the leaving group; the positive or partially positive...
. Then, acylation of the C4 hydroxyl group (acetic anhydride
Acetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula 2O. Commonly abbreviated Ac2O, it is the simplest isolatable acid anhydride and is a widely used reagent in organic synthesis...
, DMAP, pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
) resulted in acetate
Acetate
An acetate is a derivative of acetic acid. This term includes salts and esters, as well as the anion found in solution. Most of the approximately 5 billion kilograms of acetic acid produced annually in industry are used in the production of acetates, which usually take the form of polymers. In...
49. In the final steps phenyllithium
Phenyllithium
Phenyllithium is an organometallic agent with the empirical formula C6H5Li. It is most commonly used as a metalating agent in organic syntheses and a substitute for Grignard reagents for introducing phenyl groups in organic syntheses...
opened the ester group to form hydroxy carbonate 50, both TES groups were removed (HF
Hydrofluoric acid
Hydrofluoric acid is a solution of hydrogen fluoride in water. It is a valued source of fluorine and is the precursor to numerous pharmaceuticals such as fluoxetine and diverse materials such as PTFE ....
, pyr
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
) to triol 51 (baccatin III) and the C7 hydroxyl group was back-protected to 52.
 |
| Scheme 6 |
|---|
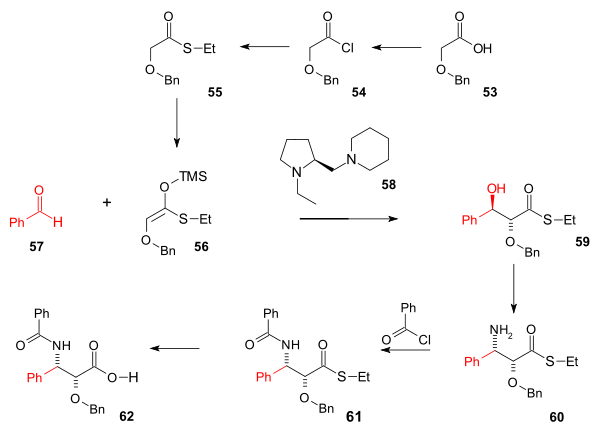
Tail synthesis
The amideAmide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
tail synthesis (scheme 7) was based on an asymmetric Aldol reaction
Aldol reaction
The aldol reaction is a powerful means of forming carbon–carbon bonds in organic chemistry.Discovered independently by Charles-Adolphe Wurtz and Alexander Porfyrevich Borodin in 1872, the reaction combines two carbonyl compounds to form a new β-hydroxy carbonyl compound...
. The starting compound is the commercially available Benzyloxyacetic acid 53 which was converted to the thio ester 55 (Ethanethiol
Ethanethiol
Ethanethiol is an organic compound with the formula CH3CH2SH. It consists of an ethyl group, CH3CH2, attached to a thiol group, SH. Its structure parallels that of ethanol, but with S instead of O. The presence of S leads to many different properties, most notably the infamous odour of EtSH...
) through the acid chloride 54 (thionyl chloride
Thionyl chloride
Thionyl chloride is an inorganic compound with the formula SOCl2. It is a reactive chemical reagent used in chlorination reactions. It is a colorless, distillable liquid at room temperature and pressure that decomposes above 140 °C. Thionyl chloride is sometimes confused with sulfuryl...
, pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
). This formed the silyl enol ether
Silyl enol ether
Silyl enol ethers in organic chemistry are a class of organic compounds that share a common functional group composed of an enolate bonded through its oxygen terminus to an organosilicon group....
55 (n-butyllithium
N-Butyllithium
n-Butyllithium is an organolithium reagent. It is widely used as a polymerization initiator in the production of elastomers such as polybutadiene or styrene-butadiene-styrene...
, trimethylsilyl chloride
Trimethylsilyl chloride
Trimethylsilyl chloride, also known as chlorotrimethylsilane is a silyl halide, with a variety of different uses in chemistry. It has the formula 3SiCl, and under standard conditions it is a colourless liquid, which is stable in the absence of water...
, Diisopropylamine
Diisopropylamine
Diisopropylamine is a secondary amine with the chemical formula 2HC-NH-CH2. It is best known as its lithium salt, lithium diisopropylamide, known as "LDA"...
) which reacted with chiral amine catalyst 58, tin triflate
Triflate
Trifluoromethanesulfonate, also known by the trivial name triflate, is a functional group with the formula CF3SO3-. The triflate group is often represented by -OTf, as opposed to -Tf...
and nBu2(OAc)2 in a Mukaiyama aldol addition
Mukaiyama aldol addition
The Mukaiyama aldol addition is an organic reaction and a type of aldol reaction between a silyl enol ether and an aldehyde catalyzed by a Lewis acid. This choice of reactants allows for a crossed aldol reaction between an aldehyde and a ketone or a different aldehyde without self-condensation of...
with benzaldehyde
Benzaldehyde
Benzaldehyde is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful. This colorless liquid has a characteristic pleasant almond-like odor...
to alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
59 with 99% anti selectivity and 96% ee
Enantiomeric excess
The enantiomeric excess of a substance is a measure of how pure it is. In this case, the impurity is the undesired enantiomer .-Definition:...
. The next step converting the alcohol group to an amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
in 60 was a Mitsunobu reaction
Mitsunobu reaction
The Mitsunobu reaction is an organic reaction that converts an alcohol into a variety of functional groups, such as an ester, using triphenylphosphine and an azodicarboxylate such as diethyl azodicarboxylate or diisopropyl azodicarboxylate . The alcohol undergoes an inversion of stereochemistry...
(hydrogen azide, DEAD, triphenylphosphine
Triphenylphosphine
Triphenylphosphine is a common organophosphorus compound with the formula P3 - often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature...
with azide reduction to amine by Ph3P). The amine group was benzoylated with benzoyl chloride
Benzoyl chloride
Benzoyl chloride, also known as benzenecarbonyl chloride, is an organochlorine compound with the formula C6H5COCl. It is a colourless, fuming liquid with an irritating odour...
(61) and hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
removes the thioether group in 62.
 |
| Scheme 7 |
|---|
Tail addition
In the final synthetic steps (scheme 8) the amide tail 62 was added to ABCD ring 52 in a esterfication catalysed by o,o'-di(2-pyridyl) thiocarbonateThiocarbonate
Thiocarbonate describes a family of anions with the general chemical formula CS3-xOx2- . Organic compounds structurally related to these anions are also called thiocarbonates....
(DPTC) and DMAP forming ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
63. The Bn protecting group was removed by hydrogenation
Hydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
using palladium hydroxide on carbon (64) and finally the TES group was removed by HF
Hydrofluoric acid
Hydrofluoric acid is a solution of hydrogen fluoride in water. It is a valued source of fluorine and is the precursor to numerous pharmaceuticals such as fluoxetine and diverse materials such as PTFE ....
and pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
to yield Taxol 65.
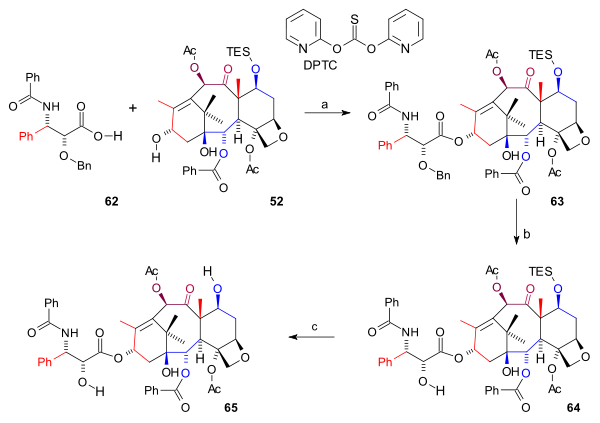 |
| Scheme 8 |
|---|
see also
- Paclitaxel total synthesis
- Danishefsky Taxol total synthesisDanishefsky Taxol total synthesisThe Danishefsky Taxol total synthesis in organic chemistry is an important third Taxol synthesis published by the group of Samuel Danishefsky in 1996...
- Holton Taxol total synthesisHolton Taxol total synthesisThe Holton Taxol total synthesis, published by Robert A. Holton and his group at Florida State University in 1994 was the first total synthesis of Taxol ....
- Kuwajima Taxol total synthesisKuwajima Taxol total synthesisThe Kuwajima Taxol total synthesis by the group of Isao Kuwajima of the Tokyo Institute of Technology is one of several efforts in taxol total synthesis published in the 1990s...
- Nicolaou Taxol total synthesisNicolaou Taxol total synthesisThe Nicolaou Taxol total synthesis, published by K. C. Nicolaou and his group in 1994 concerns the total synthesis of Taxol. This organic synthesis was included in Nicolaou's book, 'Classics in Total Synthesis'....
- Wender Taxol total synthesisWender Taxol total synthesisThe Wender Taxol total synthesis in organic chemistry describes a Taxol total synthesis by the group of Paul A. Wender at Stanford University published in 1997. This synthesis has much in common with the Holton Taxol total synthesis in that it is a linear synthesis starting from a naturally...

